Inhibition of Polo-like kinase 1 reduces beta-amyloid-induced neuronal cell death in Alzheimer's disease
- PMID: 21931181
- PMCID: PMC3227450
- DOI: 10.18632/aging.100382
Inhibition of Polo-like kinase 1 reduces beta-amyloid-induced neuronal cell death in Alzheimer's disease
Abstract
Alzheimer's disease (AD) is a progressive and fatal brain disease, but the pathogenesis of AD is still not understood. Aberrant cell-cycle re-entry of neuronal cells is emerging as a potential pathological mechanism in AD. Polo-like kinase 1 (Plk1) is an established regulator of many cell cycle-related events. Interestingly, Plk1 is present in susceptible hippocampal and cortical neurons of AD patients but not age-matched controls. However, whether Plk1 is involved in the pathogenesis of AD remains elusive. In this study, we showed that Plk1 activity is elevated in AD patient brain as indicated by the increased phosphorylation signal of p150Glued, a Plk1-specific substrate. Furthermore, we demonstrated that Plk1 is elevated during the cell-cycle re-entry of neuronal cells in an in vitro cell-culture model. Significantly, inhibition of Plk1 kinase activity or depletion of Plk1 by RNAi reduces β-amyloid (Aβ)-induced neuronal cell death. These results validate Plk1 as a possible target for AD therapy.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to declare.
Figures


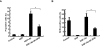
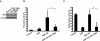
Similar articles
-
Polo-like kinase1 is required for recruitment of dynein to kinetochores during mitosis.J Biol Chem. 2011 Jun 10;286(23):20769-77. doi: 10.1074/jbc.M111.226605. Epub 2011 Apr 20. J Biol Chem. 2011. PMID: 21507953 Free PMC article.
-
Polo-like kinase 1 phosphorylation of p150Glued facilitates nuclear envelope breakdown during prophase.Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14633-8. doi: 10.1073/pnas.1006615107. Epub 2010 Aug 2. Proc Natl Acad Sci U S A. 2010. PMID: 20679239 Free PMC article.
-
Phosphoproteome-based kinase activity profiling reveals the critical role of MAP2K2 and PLK1 in neuronal autophagy.Autophagy. 2017;13(11):1969-1980. doi: 10.1080/15548627.2017.1371393. Epub 2017 Oct 4. Autophagy. 2017. PMID: 28933595 Free PMC article.
-
Targeting polo-like kinase 1 for cancer therapy.Nat Rev Cancer. 2006 Apr;6(4):321-30. doi: 10.1038/nrc1841. Nat Rev Cancer. 2006. PMID: 16557283 Review.
-
Non-mitotic functions of polo-like kinases in cancer cells.Biochim Biophys Acta Rev Cancer. 2021 Jan;1875(1):188467. doi: 10.1016/j.bbcan.2020.188467. Epub 2020 Nov 7. Biochim Biophys Acta Rev Cancer. 2021. PMID: 33171265 Review.
Cited by
-
Rat retinal transcriptome: effects of aging and AMD-like retinopathy.Cell Cycle. 2013 Jun 1;12(11):1745-61. doi: 10.4161/cc.24825. Epub 2013 May 6. Cell Cycle. 2013. PMID: 23656783 Free PMC article.
-
Neuroprotective Drug for Nerve Trauma Revealed Using Artificial Intelligence.Sci Rep. 2018 Jan 30;8(1):1879. doi: 10.1038/s41598-018-19767-3. Sci Rep. 2018. PMID: 29382857 Free PMC article.
-
Amyloid accumulation is a late event in sporadic Alzheimer's disease-like pathology in nontransgenic rats.Oncotarget. 2015 Jan 30;6(3):1396-413. doi: 10.18632/oncotarget.2751. Oncotarget. 2015. PMID: 25595891 Free PMC article.
-
Insights into the regulation of neuronal viability by nucleophosmin/B23.Exp Biol Med (Maywood). 2015 Jun;240(6):774-86. doi: 10.1177/1535370215579168. Epub 2015 Apr 22. Exp Biol Med (Maywood). 2015. PMID: 25908633 Free PMC article. Review.
-
Abortive cell cycle events in the brains of scrapie-infected hamsters with remarkable decreases of PLK3/Cdc25C and increases of PLK1/cyclin B1.Mol Neurobiol. 2013 Dec;48(3):655-68. doi: 10.1007/s12035-013-8455-1. Epub 2013 Apr 27. Mol Neurobiol. 2013. PMID: 23625313
References
-
- Zekanowski C, Wojda U. Aneuploidy, chromosomal missegregation, and cell cycle reentry in Alzheimer's disease. Acta Neurobiol Exp (Wars) 2009;69:232–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

