An in vitro evaluation of guanfacine as a substrate for P-glycoprotein
- PMID: 21931492
- PMCID: PMC3173033
- DOI: 10.2147/NDT.S24153
An in vitro evaluation of guanfacine as a substrate for P-glycoprotein
Abstract
Background: With a US Food and Drug Administration-labeled indication to treat attention-deficit/hyperactivity disorder (ADHD), the nonstimulant guanfacine has become the preferred α(2)-agonist for ADHD treatment. However, significant interindividual variability has been observed in response to guanfacine. Consequently, hypotheses of a contributing interaction with the ubiquitously expressed drug transporter, P-glycoprotein (P-gp), have arisen. We performed an in vitro study to determine if guanfacine is indeed a substrate of P-gp.
Methods: Intracellular accumulation of guanfacine was compared between P-gp expressing LLC-PK1/MDR1 cells and P-gp-negative LLC-PK1 cells to evaluate the potential interaction between P-gp and guanfacine. Cellular retention of guanfacine was analyzed using a high-performance liquid chromatographic-ultraviolet method. Rhodamine6G, a known P-gp substrate, was included in the study as a positive control.
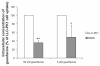
Results: At guanfacine concentrations of 50 μM and 5 μM, intracellular accumulation of guanfacine in LLC-PK1/MDR1 cells was, 35.9% ± 4.8% and 49.0% ± 28.3% respectively, of that in LLC-PK1 cells. In comparison, the concentration of rhodamine6G, the positive P-gp substrate, in LLC-PK1/MDR1 cells was only 5% of that in LLC-PK1 cells.
Conclusion: The results of the intracellular accumulation study suggest that guanfacine is, at best, a weak P-gp substrate. Therefore, it is unlikely that P-gp, or any genetic variants thereof, are a determining factor in the interindividual variability of response observed with guanfacine therapy.
Keywords: P-glycoprotein; guanfacine; intracellular uptake; substrate.
Figures
References
-
- Sorkin EM, Heel RC. Guanfacine: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of hypertension. Drugs. 1986;31(4):301–336. - PubMed
-
- Avery R, Franowicz JCS, Studholme C, van Dyck CH, Arnsten AFT. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex and improves accuracy in monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23(3):240–249. - PubMed
-
- Crenshaw TM, Kavale KA, Forness SR, Reeve RE. Attention deficit hyperactivity disorder and the efficacy of stimulant medication: A meta-analysis. In: Scruggs TE, Mastropieri MA, editors. Advances in Learning and Behavioral Disabilities. Greenwich, CT: JAI Press; 1999.
-
- Biederman J, Melmed RD, Patel A, et al. for SPD503 Study Group. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121(1):e73–e84. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

