Hsp90 governs dispersion and drug resistance of fungal biofilms
- PMID: 21931556
- PMCID: PMC3169563
- DOI: 10.1371/journal.ppat.1002257
Hsp90 governs dispersion and drug resistance of fungal biofilms
Abstract
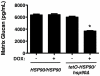
Fungal biofilms are a major cause of human mortality and are recalcitrant to most treatments due to intrinsic drug resistance. These complex communities of multiple cell types form on indwelling medical devices and their eradication often requires surgical removal of infected devices. Here we implicate the molecular chaperone Hsp90 as a key regulator of biofilm dispersion and drug resistance. We previously established that in the leading human fungal pathogen, Candida albicans, Hsp90 enables the emergence and maintenance of drug resistance in planktonic conditions by stabilizing the protein phosphatase calcineurin and MAPK Mkc1. Hsp90 also regulates temperature-dependent C. albicans morphogenesis through repression of cAMP-PKA signalling. Here we demonstrate that genetic depletion of Hsp90 reduced C. albicans biofilm growth and maturation in vitro and impaired dispersal of biofilm cells. Further, compromising Hsp90 function in vitro abrogated resistance of C. albicans biofilms to the most widely deployed class of antifungal drugs, the azoles. Depletion of Hsp90 led to reduction of calcineurin and Mkc1 in planktonic but not biofilm conditions, suggesting that Hsp90 regulates drug resistance through different mechanisms in these distinct cellular states. Reduction of Hsp90 levels led to a marked decrease in matrix glucan levels, providing a compelling mechanism through which Hsp90 might regulate biofilm azole resistance. Impairment of Hsp90 function genetically or pharmacologically transformed fluconazole from ineffectual to highly effective in eradicating biofilms in a rat venous catheter infection model. Finally, inhibition of Hsp90 reduced resistance of biofilms of the most lethal mould, Aspergillus fumigatus, to the newest class of antifungals to reach the clinic, the echinocandins. Thus, we establish a novel mechanism regulating biofilm drug resistance and dispersion and that targeting Hsp90 provides a much-needed strategy for improving clinical outcome in the treatment of biofilm infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
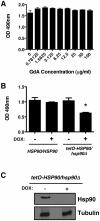

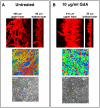
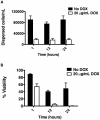

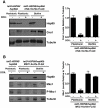


Similar articles
-
Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin.PLoS Pathog. 2009 Jul;5(7):e1000532. doi: 10.1371/journal.ppat.1000532. Epub 2009 Jul 31. PLoS Pathog. 2009. PMID: 19649312 Free PMC article.
-
PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90.PLoS Pathog. 2010 Aug 26;6(8):e1001069. doi: 10.1371/journal.ppat.1001069. PLoS Pathog. 2010. PMID: 20865172 Free PMC article.
-
The Hsp90 co-chaperone Sgt1 governs Candida albicans morphogenesis and drug resistance.PLoS One. 2012;7(9):e44734. doi: 10.1371/journal.pone.0044734. Epub 2012 Sep 6. PLoS One. 2012. PMID: 22970302 Free PMC article.
-
Heat shock protein 90 (Hsp90): A novel antifungal target against Aspergillus fumigatus.Crit Rev Microbiol. 2016;42(2):310-21. doi: 10.3109/1040841X.2014.947239. Epub 2014 Sep 22. Crit Rev Microbiol. 2016. PMID: 25243616 Review.
-
Resistance in human pathogenic yeasts and filamentous fungi: prevalence, underlying molecular mechanisms and link to the use of antifungals in humans and the environment.Dan Med J. 2016 Oct;63(10):B5288. Dan Med J. 2016. PMID: 27697142 Review.
Cited by
-
Synergistic Effect of Plant Compounds in Combination with Conventional Antimicrobials against Biofilm of Staphylococcus aureus, Pseudomonas aeruginosa, and Candida spp.Pharmaceuticals (Basel). 2023 Oct 30;16(11):1531. doi: 10.3390/ph16111531. Pharmaceuticals (Basel). 2023. PMID: 38004397 Free PMC article. Review.
-
Mapping the Hsp90 Genetic Network Reveals Ergosterol Biosynthesis and Phosphatidylinositol-4-Kinase Signaling as Core Circuitry Governing Cellular Stress.PLoS Genet. 2016 Jun 24;12(6):e1006142. doi: 10.1371/journal.pgen.1006142. eCollection 2016 Jun. PLoS Genet. 2016. PMID: 27341673 Free PMC article.
-
Candida albicans Biofilm Inhibition by Ethnobotanicals and Ethnobotanically-Synthesized Gold Nanoparticles.Front Microbiol. 2021 May 24;12:665113. doi: 10.3389/fmicb.2021.665113. eCollection 2021. Front Microbiol. 2021. PMID: 34108950 Free PMC article.
-
Lysine deacetylases Hda1 and Rpd3 regulate Hsp90 function thereby governing fungal drug resistance.Cell Rep. 2012 Oct 25;2(4):878-88. doi: 10.1016/j.celrep.2012.08.035. Epub 2012 Oct 4. Cell Rep. 2012. PMID: 23041319 Free PMC article.
-
Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system.Microbiol Mol Biol Rev. 2012 Jun;76(2):115-58. doi: 10.1128/MMBR.05018-11. Microbiol Mol Biol Rev. 2012. PMID: 22688810 Free PMC article. Review.
References
-
- Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53. - PubMed
-
- McNeil MM, Nash SL, Hajjeh RA, Phelan MA, Conn LA, et al. Trends in mortality due to invasive mycotic diseases in the United States, 1980–1997. Clin Infect Dis. 2001;33:641–647. - PubMed
-
- Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, et al. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41:1232–1239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

