Genetic effects at pleiotropic loci are context-dependent with consequences for the maintenance of genetic variation in populations
- PMID: 21931559
- PMCID: PMC3169520
- DOI: 10.1371/journal.pgen.1002256
Genetic effects at pleiotropic loci are context-dependent with consequences for the maintenance of genetic variation in populations
Abstract
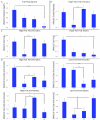
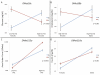
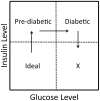
Context-dependent genetic effects, including genotype-by-environment and genotype-by-sex interactions, are a potential mechanism by which genetic variation of complex traits is maintained in populations. Pleiotropic genetic effects are also thought to play an important role in evolution, reflecting functional and developmental relationships among traits. We examine context-dependent genetic effects at pleiotropic loci associated with normal variation in multiple metabolic syndrome (MetS) components (obesity, dyslipidemia, and diabetes-related traits). MetS prevalence is increasing in Western societies and, while environmental in origin, presents substantial variation in individual response. We identify 23 pleiotropic MetS quantitative trait loci (QTL) in an F(16) advanced intercross between the LG/J and SM/J inbred mouse strains (Wustl:LG,SM-G16; n = 1002). Half of each family was fed a high-fat diet and half fed a low-fat diet; and additive, dominance, and parent-of-origin imprinting genotypic effects were examined in animals partitioned into sex, diet, and sex-by-diet cohorts. We examine the context-dependency of the underlying additive, dominance, and imprinting genetic effects of the traits associated with these pleiotropic QTL. Further, we examine sequence polymorphisms (SNPs) between LG/J and SM/J as well as differential expression of positional candidate genes in these regions. We show that genetic associations are different in different sex, diet, and sex-by-diet settings. We also show that over- or underdominance and ecological cross-over interactions for single phenotypes may not be common, however multidimensional synthetic phenotypes at loci with pleiotropic effects can produce situations that favor the maintenance of genetic variation in populations. Our findings have important implications for evolution and the notion of personalized medicine.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The importance of context to the genetic architecture of diabetes-related traits is revealed in a genome-wide scan of a LG/J × SM/J murine model.Mamm Genome. 2011 Apr;22(3-4):197-208. doi: 10.1007/s00335-010-9313-3. Epub 2011 Jan 6. Mamm Genome. 2011. PMID: 21210123 Free PMC article.
-
Diet-dependent genetic and genomic imprinting effects on obesity in mice.Obesity (Silver Spring). 2011 Jan;19(1):160-70. doi: 10.1038/oby.2010.141. Epub 2010 Jun 10. Obesity (Silver Spring). 2011. PMID: 20539295 Free PMC article.
-
Genetic, epigenetic, and gene-by-diet interaction effects underlie variation in serum lipids in a LG/JxSM/J murine model.J Lipid Res. 2010 Oct;51(10):2976-84. doi: 10.1194/jlr.M006957. Epub 2010 Jul 2. J Lipid Res. 2010. PMID: 20601649 Free PMC article.
-
Pleiotropy and the evolution of floral integration.New Phytol. 2016 Jan;209(1):80-5. doi: 10.1111/nph.13583. Epub 2015 Jul 30. New Phytol. 2016. PMID: 26224529 Review.
-
Genomic imprinting effects on complex traits: a phenotype-based perspective.Epigenetics. 2008 Nov;3(6):295-9. doi: 10.4161/epi.3.6.7257. Epub 2008 Nov 24. Epigenetics. 2008. PMID: 19029803 Review.
Cited by
-
A new statistical framework for genetic pleiotropic analysis of high dimensional phenotype data.BMC Genomics. 2016 Nov 7;17(1):881. doi: 10.1186/s12864-016-3169-1. BMC Genomics. 2016. PMID: 27821073 Free PMC article.
-
Parent-of-Origin Effects of the APOB Gene on Adiposity in Young Adults.PLoS Genet. 2015 Oct 9;11(10):e1005573. doi: 10.1371/journal.pgen.1005573. eCollection 2015 Oct. PLoS Genet. 2015. PMID: 26451733 Free PMC article.
-
The Sleep Inbred Panel, a Collection of Inbred Drosophila melanogaster with Extreme Long and Short Sleep Duration.G3 (Bethesda). 2018 Aug 30;8(9):2865-2873. doi: 10.1534/g3.118.200503. G3 (Bethesda). 2018. PMID: 29991508 Free PMC article.
-
The Interaction of Genetic Background and Mutational Effects in Regulation of Mouse Craniofacial Shape.G3 (Bethesda). 2017 May 5;7(5):1439-1450. doi: 10.1534/g3.117.040659. G3 (Bethesda). 2017. PMID: 28280213 Free PMC article.
-
QTL mapping in outbred populations: successes and challenges.Physiol Genomics. 2014 Feb 1;46(3):81-90. doi: 10.1152/physiolgenomics.00127.2013. Epub 2013 Dec 10. Physiol Genomics. 2014. PMID: 24326347 Free PMC article. Review.
References
-
- Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370:1929–1938. - PubMed
-
- Heymsfield SB. How large is the energy gap that accounts for the obesity epidemic? Am J Clin Nutr. 2009;89:1717–1718. - PubMed
-
- Musani SK, Erickson S, Allison DB. Obesity--still highly heritable after all these years. Am J Clin Nutr. 2008;87:275–276. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

