Lithium suppresses astrogliogenesis by neural stem and progenitor cells by inhibiting STAT3 pathway independently of glycogen synthase kinase 3 beta
- PMID: 21931595
- PMCID: PMC3170293
- DOI: 10.1371/journal.pone.0023341
Lithium suppresses astrogliogenesis by neural stem and progenitor cells by inhibiting STAT3 pathway independently of glycogen synthase kinase 3 beta
Abstract
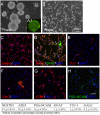
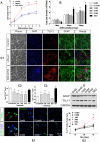
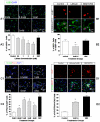
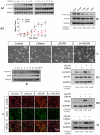
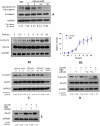
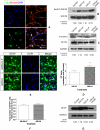
Transplanted neural stem and progenitor cells (NSCs) produce mostly astrocytes in injured spinal cords. Lithium stimulates neurogenesis by inhibiting GSK3b (glycogen synthetase kinase 3-beta) and increasing WNT/beta catenin. Lithium suppresses astrogliogenesis but the mechanisms were unclear. We cultured NSCs from subventricular zone of neonatal rats and showed that lithium reduced NSC production of astrocytes as well as proliferation of glia restricted progenitor (GRP) cells. Lithium strongly inhibited STAT3 (signal transducer and activator of transcription 3) activation, a messenger system known to promote astrogliogenesis and cancer. Lithium abolished STAT3 activation and astrogliogenesis induced by a STAT3 agonist AICAR (5-aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside), suggesting that lithium suppresses astrogliogenesis by inhibiting STAT3. GSK3β inhibition either by a specific GSK3β inhibitor SB216763 or overexpression of GID5-6 (GSK3β Interaction Domain aa380 to 404) did not suppress astrogliogenesis and GRP proliferation. GSK3β inhibition also did not suppress STAT3 activation. Together, these results indicate that lithium inhibits astrogliogenesis through non-GSK3β-mediated inhibition of STAT. Lithium may increase efficacy of NSC transplants by increasing neurogenesis and reducing astrogliogenesis. Our results also may explain the strong safety record of lithium treatment of manic depression. Millions of people take high-dose (>1 gram/day) lithium carbonate for a lifetime. GSK3b inhibition increases WNT/beta catenin, associated with colon and other cancers. STAT3 inhibition may reduce risk for cancer.
Conflict of interest statement
Figures
References
-
- Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, et al. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167:48–58. - PubMed
-
- Nakamura M, Okada S, Toyama Y, Okano H. Role of IL-6 in spinal cord injury in a mouse model. Clin Rev Allergy Immunol. 2005;28:197–204. - PubMed
-
- Mukaino M, Nakamura M, Okada S, Toyama Y, Liu M, et al. [Role of IL-6 in regulation of inflammation and stem cell differentiation in CNS trauma]. Nihon Rinsho Meneki Gakkai Kaishi. 2008;31:93–98. - PubMed
-
- Lee MY, Kim CJ, Shin SL, Moon SH, Chun MH. Increased ciliary neurotrophic factor expression in reactive astrocytes following spinal cord injury in the rat. Neurosci Lett. 1998;255:79–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

