A soluble factor from Trypanosoma cruzi inhibits transforming growth factor-ß-induced MAP kinase activation and gene expression in dermal fibroblasts
- PMID: 21931601
- PMCID: PMC3169535
- DOI: 10.1371/journal.pone.0023482
A soluble factor from Trypanosoma cruzi inhibits transforming growth factor-ß-induced MAP kinase activation and gene expression in dermal fibroblasts
Abstract
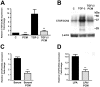
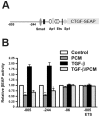
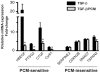
The protozoan parasite Trypanosoma cruzi, which causes human Chagas' disease, exerts a variety of effects on host extracellular matrix (ECM) including proteolytic degradation of collagens and dampening of ECM gene expression. Exposure of primary human dermal fibroblasts to live infective T. cruzi trypomastigotes or their shed/secreted products results in a rapid down-regulation of the fibrogenic genes collagenIα1, fibronectin and connective tissue growth factor (CTGF/CCN2). Here we demonstrate the ability of a secreted/released T. cruzi factor to antagonize ctgf/ccn2 expression in dermal fibroblasts in response to TGF-ß, lysophosphatidic acid or serum, where agonist-induced phosphorylation of the mitogen-activated protein (MAP) kinases Erk1/2, p38 and JNK was also inhibited. Global analysis of gene expression in dermal fibroblasts identified a discrete subset of TGF-ß-inducible genes involved in cell proliferation, wound repair, and immune regulation that are inhibited by T. cruzi secreted/released factors, where the genes exhibiting the highest sensitivity to T. cruzi are known to be regulated by MAP kinase-activated transcription factors. Consistent with this observation, the Ets-family transcription factor binding site in the proximal promoter region of the ctgf/ccn2 gene (-91 bp to -84 bp) was shown to be required for T. cruzi-mediated down-regulation of ctgf/ccn2 reporter expression. The cumulative data suggest a model in which T. cruzi-derived molecules secreted/released early in the infective process dampen MAP kinase signaling and the activation of transcription factors that regulate expression of fibroblast genes involved in wound repair and tissue remodelling, including ctgf/ccn2. These findings have broader implications for local modulation of ECM synthesis/remodelling by T. cruzi during the early establishment of infection in the mammalian host and highlight the potential for pathogen-derived molecules to be exploited as tools to modulate the fibrogenic response.
Conflict of interest statement
Figures


Similar articles
-
Inhibition of host connective tissue growth factor expression: a novel Trypanosoma cruzi-mediated response.FASEB J. 2004 Nov;18(14):1625-35. doi: 10.1096/fj.04-1554com. FASEB J. 2004. PMID: 15522908
-
C-Jun-NH2-terminal kinase mediates expression of connective tissue growth factor induced by transforming growth factor-beta1 in human lung fibroblasts.Am J Respir Cell Mol Biol. 2003 Jun;28(6):754-61. doi: 10.1165/rcmb.4892. Am J Respir Cell Mol Biol. 2003. PMID: 12760970
-
Inhibition of connective tissue growth factor/CCN2 expression in human dermal fibroblasts by interleukin-1alpha and beta.J Cell Biochem. 2010 Aug 1;110(5):1226-33. doi: 10.1002/jcb.22637. J Cell Biochem. 2010. PMID: 20544797
-
Regulation of hepatic stellate cells by connective tissue growth factor.Front Biosci (Landmark Ed). 2012 Jun 1;17(7):2495-507. doi: 10.2741/4067. Front Biosci (Landmark Ed). 2012. PMID: 22652794 Review.
-
Extracellular matrix-derived peptides in tissue remodeling and fibrosis.Matrix Biol. 2020 Sep;91-92:176-187. doi: 10.1016/j.matbio.2020.04.006. Epub 2020 May 8. Matrix Biol. 2020. PMID: 32438055 Free PMC article. Review.
Cited by
-
MEK/ERK inhibitors: proof-of-concept studies in lung fibrosis.J Cell Commun Signal. 2012 Mar;6(1):59-60. doi: 10.1007/s12079-011-0156-9. Epub 2011 Dec 1. J Cell Commun Signal. 2012. PMID: 22131200 Free PMC article.
-
Getting out of a sticky situation: targeting the myofibroblast in scleroderma.Open Rheumatol J. 2012;6:163-9. doi: 10.2174/1874312901206010163. Epub 2012 Jun 15. Open Rheumatol J. 2012. PMID: 22802915 Free PMC article.
-
Differential Role of TGF-β in Extracellular Matrix Regulation During Trypanosoma cruzi-Host Cell Interaction.Int J Mol Sci. 2019 Sep 29;20(19):4836. doi: 10.3390/ijms20194836. Int J Mol Sci. 2019. PMID: 31569452 Free PMC article.
-
Trypanosoma cruzi dysregulates expression profile of piRNAs in primary human cardiac fibroblasts during early infection phase.Front Cell Infect Microbiol. 2023 Mar 2;13:1083379. doi: 10.3389/fcimb.2023.1083379. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36936778 Free PMC article.
-
Topical Mentha piperita Effects on Cutaneous Wound Healing: A Study on TGF-β Expression and Clinical Outcomes.World J Plast Surg. 2022 Mar;11(1):86-96. doi: 10.52547/wjps.11.1.86. World J Plast Surg. 2022. PMID: 35592219 Free PMC article.
References
-
- Yoshida N. Trypanosoma cruzi infection by oral route: how the interplay between parasite and host components modulates infectivity. Parasitol Int. 2008;57:105–109. - PubMed
-
- Mott GA, Burleigh BA. The role of host cell lysosomes in Trypanosoma cruzi invasion. Subcell Biochem. 2008;47:165–173. - PubMed
-
- de Avalos SV, Blader IJ, Fisher M, Boothroyd JC, Burleigh BA. Immediate/Early Response to Trypanosoma cruzi Infection Involves Minimal Modulation of Host Cell Transcription. Journal of Biological Chemistry. 2002;277:639–644. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

