Key role of polyphosphoinositides in dynamics of fusogenic nuclear membrane vesicles
- PMID: 21931619
- PMCID: PMC3169559
- DOI: 10.1371/journal.pone.0023859
Key role of polyphosphoinositides in dynamics of fusogenic nuclear membrane vesicles
Abstract
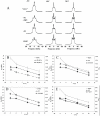
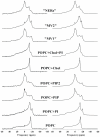
The role of phosphoinositides has been thoroughly described in many signalling and membrane trafficking events but their function as modulators of membrane structure and dynamics in membrane fusion has not been investigated. We have reconstructed models that mimic the composition of nuclear envelope precursor membranes with naturally elevated amounts of phosphoinositides. These fusogenic membranes (membrane vesicle 1(MV1) and nuclear envelope remnants (NER) are critical for the assembly of the nuclear envelope. Phospholipids, cholesterol, and polyphosphoinositides, with polyunsaturated fatty acid chains that were identified in the natural nuclear membranes by lipid mass spectrometry, have been used to reconstruct complex model membranes mimicking nuclear envelope precursor membranes. Structural and dynamic events occurring in the membrane core and at the membrane surface were monitored by solid-state deuterium and phosphorus NMR. "MV1-like" (PC∶PI∶PIP∶PIP(2), 30∶20∶18∶12, mol%) membranes that exhibited high levels of PtdIns, PtdInsP and PtdInsP(2) had an unusually fluid membrane core (up to 20% increase, compared to membranes with low amounts of phosphoinositides to mimic the endoplasmic reticulum). "NER-like" (PC∶CH∶PI∶PIP∶PIP(2), 28∶42∶16∶7∶7, mol%) membranes containing high amounts of both cholesterol and phosphoinositides exhibited liquid-ordered phase properties, but with markedly lower rigidity (10-15% decrease). Phosphoinositides are the first lipids reported to counterbalance the ordering effect of cholesterol. At the membrane surface, phosphoinositides control the orientation dynamics of other lipids in the model membranes, while remaining unchanged themselves. This is an important finding as it provides unprecedented mechanistic insight into the role of phosphoinositides in membrane dynamics. Biological implications of our findings and a model describing the roles of fusogenic membrane vesicles are proposed.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

