Different toll-like receptor stimuli have a profound impact on cytokines required to break tolerance and induce autoimmunity
- PMID: 21931625
- PMCID: PMC3171407
- DOI: 10.1371/journal.pone.0023940
Different toll-like receptor stimuli have a profound impact on cytokines required to break tolerance and induce autoimmunity
Abstract
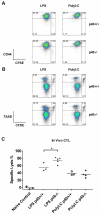
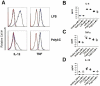
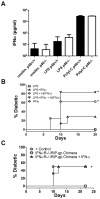
Although toll-like receptor (TLR) signals are critical for promoting antigen presenting cell maturation, it remains unclear how stimulation via different TLRs influence dendritic cell (DC) function and the subsequent adaptive response in vivo. Furthermore, the relationship between TLR-induced cytokine production by DCs and the consequences on the induction of a functional immune response is not clear. We have established a murine model to examine whether TLR3 or TLR4 mediated DC maturation has an impact on the cytokines required to break tolerance and induce T-cell-mediated autoimmunity. Our study demonstrates that IL-12 is not absolutely required for the induction of a CD8 T-cell-mediated tissue specific immune response, but rather the requirement for IL-12 is determined by the stimuli used to mature the DCs. Furthermore, we found that IFNα is a critical pathogenic component of the cytokine milieu that circumvents the requirement for IL-12 in the induction of autoimmunity. These studies illustrate how different TLR stimuli have an impact on DC function and the induction of immunity.
Conflict of interest statement
Figures

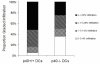

Similar articles
-
miR-155 Upregulation in Dendritic Cells Is Sufficient To Break Tolerance In Vivo by Negatively Regulating SHIP1.J Immunol. 2015 Nov 15;195(10):4632-40. doi: 10.4049/jimmunol.1302941. Epub 2015 Oct 7. J Immunol. 2015. PMID: 26447227
-
IFN-alpha skews monocyte differentiation into Toll-like receptor 7-expressing dendritic cells with potent functional activities.J Immunol. 2003 Oct 1;171(7):3385-93. doi: 10.4049/jimmunol.171.7.3385. J Immunol. 2003. PMID: 14500632
-
IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells.J Immunol. 2004 Dec 15;173(12):7548-55. doi: 10.4049/jimmunol.173.12.7548. J Immunol. 2004. PMID: 15585882
-
Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells.Eur J Immunol. 2009 Sep;39(9):2325-30. doi: 10.1002/eji.200939548. Eur J Immunol. 2009. PMID: 19701895 Review.
-
Opposing effects of Toll-like receptor stimulation induce autoimmunity or tolerance.Trends Immunol. 2007 Feb;28(2):74-9. doi: 10.1016/j.it.2006.12.006. Epub 2007 Jan 2. Trends Immunol. 2007. PMID: 17197239 Review.
Cited by
-
ARIH2 is essential for embryogenesis, and its hematopoietic deficiency causes lethal activation of the immune system.Nat Immunol. 2013 Jan;14(1):27-33. doi: 10.1038/ni.2478. Epub 2012 Nov 25. Nat Immunol. 2013. PMID: 23179078
-
Hypoxia-inducible factor 1 alpha limits dendritic cell stimulation of CD8 T cell immunity.PLoS One. 2020 Dec 31;15(12):e0244366. doi: 10.1371/journal.pone.0244366. eCollection 2020. PLoS One. 2020. PMID: 33382742 Free PMC article.
-
The effects of TLR activation on T-cell development and differentiation.Clin Dev Immunol. 2012;2012:836485. doi: 10.1155/2012/836485. Epub 2012 Jun 7. Clin Dev Immunol. 2012. PMID: 22737174 Free PMC article. Review.
-
The biography of the immune system and the control of cancer: from St Peregrine to contemporary vaccination strategies.BMC Cancer. 2014 Aug 16;14:595. doi: 10.1186/1471-2407-14-595. BMC Cancer. 2014. PMID: 25128300 Free PMC article. Review.
-
NK Cells Regulate CD8+ T Cell Mediated Autoimmunity.Front Cell Infect Microbiol. 2020 Feb 13;10:36. doi: 10.3389/fcimb.2020.00036. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32117809 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

