Triptolide (TPL) inhibits global transcription by inducing proteasome-dependent degradation of RNA polymerase II (Pol II)
- PMID: 21931633
- PMCID: PMC3172214
- DOI: 10.1371/journal.pone.0023993
Triptolide (TPL) inhibits global transcription by inducing proteasome-dependent degradation of RNA polymerase II (Pol II)
Abstract
Triptolide (TPL), a key biologically active component of the Chinese medicinal herb Tripterygium wilfordii Hook. f., has potent anti-inflammation and anti-cancer activities. Its anti-proliferative and pro-apoptotic effects have been reported to be related to the inhibition of Nuclear Factor κB (NF-κB) and Nuclear Factor of Activated T-cells (NFAT) mediated transcription and suppression of HSP70 expression. The direct targets and precise mechanisms that are responsible for the gene expression inhibition, however, remain unknown. Here, we report that TPL inhibits global gene transcription by inducing proteasome-dependent degradation of the largest subunit of RNA polymerase II (Rpb1) in cancer cells. In the presence of proteosome inhibitor MG132, TPL treatment causes hyperphosphorylation of Rpb1 by activation of upstream protein kinases such as Positive Transcription Elongation Factor b (P-TEFb) in a time and dose dependent manner. Also, we observe that short time incubation of TPL with cancer cells induces DNA damage. In conclusion, we propose a new mechanism of how TPL works in killing cancer. TPL inhibits global transcription in cancer cells by induction of phosphorylation and subsequent proteasome-dependent degradation of Rpb1 resulting in global gene transcription, which may explain the high potency of TPL in killing cancer.
Conflict of interest statement
Figures
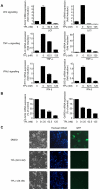



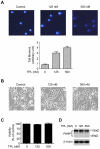
References
-
- Efferth T, Li PC, Konkimalla VS, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 2007;13:353–361. - PubMed
-
- Bao X, Cui J, Wu Y, Han X, Gao C, et al. The roles of endogenous reactive oxygen species and nitric oxide in triptolide-induced apoptotic cell death in macrophages. J Mol Med. 2007;85:85–98. - PubMed
-
- Fidler JM, Li K, Chung C, Wei K, Ross JA, et al. PG490-88, a derivative of triptolide, causes tumor regression and sensitizes tumors to chemotherapy. Mol Cancer Ther. 2003;2:855–862. - PubMed
-
- Kiviharju TM, Lecane PS, Sellers RG, Peehl DM. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin Cancer Res. 2002;8:2666–2674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

