Type 2 diabetes is associated with altered NF-κB DNA binding activity, JNK phosphorylation, and AMPK phosphorylation in skeletal muscle after LPS
- PMID: 21931634
- PMCID: PMC3172218
- DOI: 10.1371/journal.pone.0023999
Type 2 diabetes is associated with altered NF-κB DNA binding activity, JNK phosphorylation, and AMPK phosphorylation in skeletal muscle after LPS
Abstract
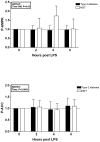
Systemic inflammation is often associated with impaired glucose metabolism. We therefore studied the activation of inflammatory pathway intermediates that interfere with glucose uptake during systemic inflammation by applying a standardised inflammatory stimulus in vivo. After ethical approval, informed consent and a thorough physical examination, 10 patients with type 2 diabetes and 10 participants with normal glucose tolerance (NGT) were given an intravenous bolus of E. coli lipopolysaccharide (LPS) of 0.3 ng/kg. Skeletal muscle biopsies and plasma were obtained at baseline and two, four and six hours after LPS. Nuclear factor (NF)-κB p65 DNA binding activity measured by ELISA, tumor necrosis factor-α and interleukin-6 mRNA expression analysed by real time reverse transcription polymerase chain reaction, and abundance of inhibitor of NF-κB (IκB)α, phosphorylated c-Jun-N-terminal kinase (JNK), AMP-activated protein kinase (AMPK), and acetyl-CoA carboxylase measured by Western blotting were detected in muscle biopsy samples. Relative to subjects with NGT, patients with type 2 diabetes exhibited a more pronounced increase in NF-κB binding activity and JNK phosphorylation after LPS, whereas skeletal muscle cytokine mRNA expression did not differ significantly between groups. AMPK phosphorylation increased in volunteers with NGT, but not in those with diabetes. The present findings indicate that pathways regulating glucose uptake in skeletal muscle may be involved in the development of inflammation-associated hyperglycemia. Patients with type 2 diabetes exhibit changes in these pathways, which may ultimately render such patients more prone to develop dysregulated glucose disposal in the context of systemic inflammation.
Trial registration: ClinicalTrials.gov NCT00412906.
Conflict of interest statement
Figures


References
-
- Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–1292. - PubMed
-
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. - PubMed
-
- Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291–300. - PubMed
-
- Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. - PubMed
-
- Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, et al. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–2945. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

