Sirtinol treatment reduces inflammation in human dermal microvascular endothelial cells
- PMID: 21931678
- PMCID: PMC3171404
- DOI: 10.1371/journal.pone.0024307
Sirtinol treatment reduces inflammation in human dermal microvascular endothelial cells
Erratum in
- PLoS One. 2011;6(10). doi:10.1371/annotation/0174b439-c62b-42e8-a420-3defbfe5c8e9
Abstract
Histone deacetylases (HDAC) are key enzymes in the epigenetic control of gene expression. Recently, inhibitors of class I and class II HDAC have been successfully employed for the treatment of different inflammatory diseases such as rheumatoid arthritis, colitis, airway inflammation and asthma. So far, little is known so far about a similar therapeutic effect of inhibitors specifically directed against sirtuins, the class III HDAC. In this study, we investigated the expression and localization of endogenous sirtuins in primary human dermal microvascular endothelial cells (HDMEC), a cell type playing a key role in the development and maintenance of skin inflammation. We then examined the biological activity of sirtinol, a specific sirtuin inhibitor, in HDMEC response to pro-inflammatory cytokines. We found that, even though sirtinol treatment alone affected only long-term cell proliferation, it diminishes HDMEC inflammatory responses to tumor necrosis factor (TNF)α and interleukin (IL)-1β. In fact, sirtinol significantly reduced membrane expression of adhesion molecules in TNFã- or IL-1β-stimulated cells, as well as the amount of CXCL10 and CCL2 released by HDMEC following TNFα treatment. Notably, sirtinol drastically decreased monocyte adhesion on activated HDMEC. Using selective inhibitors for Sirt1 and Sirt2, we showed a predominant involvement of Sirt1 inhibition in the modulation of adhesion molecule expression and monocyte adhesion on activated HDMEC. Finally, we demonstrated the in vivo expression of Sirt1 in the dermal vessels of normal and psoriatic skin. Altogether, these findings indicated that sirtuins may represent a promising therapeutic target for the treatment of inflammatory skin diseases characterized by a prominent microvessel involvement.
Conflict of interest statement
Figures
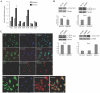


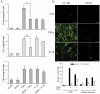
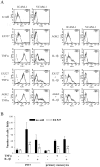
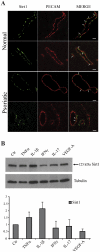
References
-
- Guarente L, Picard F. Calorie restriction-the SIR2 connection. Cell. 2005;120:473–482. - PubMed
-
- Donmez G, Guarente L. Aging and disease: connections to sirtuins. Aging Cell. 2010;9:285–290. - PubMed
-
- Frye RA. Phylogenic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Comm. 2000;273:793–798. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

