Sequential alterations in catabolic and anabolic gene expression parallel pathological changes during progression of monoiodoacetate-induced arthritis
- PMID: 21931681
- PMCID: PMC3172226
- DOI: 10.1371/journal.pone.0024320
Sequential alterations in catabolic and anabolic gene expression parallel pathological changes during progression of monoiodoacetate-induced arthritis
Abstract
Chronic inflammation is one of the major causes of cartilage destruction in osteoarthritis. Here, we systematically analyzed the changes in gene expression associated with the progression of cartilage destruction in monoiodoacetate-induced arthritis (MIA) of the rat knee. Sprague Dawley female rats were given intra-articular injection of monoiodoacetate in the knee. The progression of MIA was monitored macroscopically, microscopically and by micro-computed tomography. Grade 1 damage was observed by day 5 post-monoiodoacetate injection, progressively increasing to Grade 2 by day 9, and to Grade 3-3.5 by day 21. Affymetrix GeneChip was utilized to analyze the transcriptome-wide changes in gene expression, and the expression of salient genes was confirmed by real-time-PCR. Functional networks generated by Ingenuity Pathways Analysis (IPA) from the microarray data correlated the macroscopic/histologic findings with molecular interactions of genes/gene products. Temporal changes in gene expression during the progression of MIA were categorized into five major gene clusters. IPA revealed that Grade 1 damage was associated with upregulation of acute/innate inflammatory responsive genes (Cluster I) and suppression of genes associated with musculoskeletal development and function (Cluster IV). Grade 2 damage was associated with upregulation of chronic inflammatory and immune trafficking genes (Cluster II) and downregulation of genes associated with musculoskeletal disorders (Cluster IV). The Grade 3 to 3.5 cartilage damage was associated with chronic inflammatory and immune adaptation genes (Cluster III). These findings suggest that temporal regulation of discrete gene clusters involving inflammatory mediators, receptors, and proteases may control the progression of cartilage destruction. In this process, IL-1β, TNF-α, IL-15, IL-12, chemokines, and NF-κB act as central nodes of the inflammatory networks, regulating catabolic processes. Simultaneously, upregulation of asporin, and downregulation of TGF-β complex, SOX-9, IGF and CTGF may be central to suppress matrix synthesis and chondrocytic anabolic activities, collectively contributing to the progression of cartilage destruction in MIA.
Conflict of interest statement
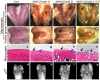
Figures








References
-
- Pleis J, Lucas J, Ward B. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital Health Stat. 2009;10 - PubMed
-
- Loeser RF. Translational research in osteoarthritis–from molecules to animals to humans. J Musculoskelet Neuronal Interact. 2008;8:301–302. - PubMed
-
- Vincent TL, Saklatvala J. Is the response of cartilage to injury relevant to osteoarthritis? Arthritis Rheum. 2008;58:1207–1210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

