Specific interaction of Gαi3 with the Oa1 G-protein coupled receptor controls the size and density of melanosomes in retinal pigment epithelium
- PMID: 21931697
- PMCID: PMC3169599
- DOI: 10.1371/journal.pone.0024376
Specific interaction of Gαi3 with the Oa1 G-protein coupled receptor controls the size and density of melanosomes in retinal pigment epithelium
Abstract
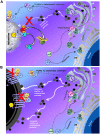
Background: Ocular albinism type 1, an X-linked disease characterized by the presence of enlarged melanosomes in the retinal pigment epithelium (RPE) and abnormal crossing of axons at the optic chiasm, is caused by mutations in the OA1 gene. The protein product of this gene is a G-protein-coupled receptor (GPCR) localized in RPE melanosomes. The Oa1-/- mouse model of ocular albinism reproduces the human disease. Oa1 has been shown to immunoprecipitate with the Gαi subunit of heterotrimeric G proteins from human skin melanocytes. However, the Gαi subfamily has three highly homologous members, Gαi1, Gαi2 and Gαi3 and it is possible that one or more of them partners with Oa1. We had previously shown by in-vivo studies that Gαi3-/- and Oa1-/- mice have similar RPE phenotype and decussation patterns. In this paper we analyze the specificity of the Oa1-Gαi interaction.
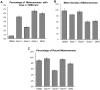
Methodology: By using the genetic mouse models Gαi1-/-, Gαi2-/-, Gαi3-/- and the double knockout Gαi1-/-, Gαi3-/- that lack functional Gαi1, Gαi2, Gαi3, or both Gαi1 and Gαi3 proteins, respectively, we show that Gαi3 is critical for the maintenance of a normal melanosomal phenotype and that its absence is associated with changes in melanosomal size and density. GST-pull-down and immunoprecipitation assays conclusively demonstrate that Gαi3 is the only Gαi that binds to Oa1. Western blots show that Gαi3 expression is barely detectable in the Oa1-/- RPE, strongly supporting a previously unsuspected role for Gαi3 in melanosomal biogenesis.
Conclusion: Our results identify the Oa1 transducer Gαi3 as the first downstream component in the Oa1 signaling pathway.
Conflict of interest statement
Figures

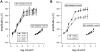



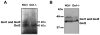
References
-
- King RC, Stansfield WD, Mulligan PK. A Dictionary of Genetics, Seventh Edition. Oxford University Press; 2006.
-
- Oetting WS, Summers CG, King RA. Albinism and the associated ocular defects. Metab Pediatr Syst Ophthalmol. 1994;17:5–9. - PubMed
-
- Garner A, Jay BS. Macromelanosomes in X-linked ocular albinism. Histopathology. 1980;4:243–54. - PubMed
-
- O'Donnell FE, Jr, Hambrick GW, Jr, Green WR, Iliff WJ, Stone DL. X-linked ocular albinism: An oculocutaneous macromelanosomal disorder. Arch Ophthalmol. 1976;94:1883–92. - PubMed
-
- Schiaffino MV, Tacchetti C. The ocular albinism type 1 (OA1) protein and the evidence for an intracellular signal transduction system involved in melanosome biogenesis. Pigment Cell Res. 2005;18:227–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

