Cyclic AMP control measured in two compartments in HEK293 cells: phosphodiesterase K(M) is more important than phosphodiesterase localization
- PMID: 21931705
- PMCID: PMC3169605
- DOI: 10.1371/journal.pone.0024392
Cyclic AMP control measured in two compartments in HEK293 cells: phosphodiesterase K(M) is more important than phosphodiesterase localization
Abstract
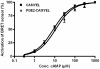
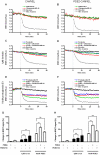
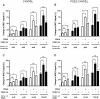
The intracellular second messenger cyclic AMP (cAMP) is degraded by phosphodiesterases (PDE). The knowledge of individual families and subtypes of PDEs is considerable, but how the different PDEs collaborate in the cell to control a cAMP signal is still not fully understood. In order to investigate compartmentalized cAMP signaling, we have generated a membrane-targeted variant of the cAMP Bioluminiscence Resonance Energy Transfer (BRET) sensor CAMYEL and have compared intracellular cAMP measurements with it to measurements with the cytosolic BRET sensor CAMYEL in HEK293 cells. With these sensors we observed a slightly higher cAMP response to adenylyl cyclase activation at the plasma membrane compared to the cytosol, which is in accordance with earlier results from Fluorescence Resonance Energy Transfer (FRET) sensors. We have analyzed PDE activity in fractionated lysates from HEK293 cells using selective PDE inhibitors and have identified PDE3 and PDE10A as the major membrane-bound PDEs and PDE4 as the major cytosolic PDE. Inhibition of membrane-bound or cytosolic PDEs can potentiate the cAMP response to adenylyl cyclase activation, but we see no significant difference between the potentiation of the cAMP response at the plasma membrane and in cytosol when membrane-bound and cytosolic PDEs are inhibited. When different levels of stimulation were tested, we found that PDEs 3 and 10 are mainly responsible for cAMP degradation at low intracellular cAMP concentrations, whereas PDE4 is more important for control of cAMP at higher concentrations.
Conflict of interest statement
Figures
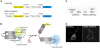
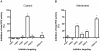
References
-
- Beavo JA, Brunton LL. Cyclic nucleotide research – still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. - PubMed
-
- Chin KV, Yang WL, Ravatn R, Kita T, Reitman E, et al. Reinventing the wheel of cyclic AMP: novel mechanisms of cAMP signaling. Ann N Y Acad Sci. 2002;968:49–64. - PubMed
-
- Cooper DM. Compartmentalization of adenylate cyclase and cAMP signalling. Biochem Soc Trans. 2005;33:1319–1322. - PubMed
-
- McKnight GS. Cyclic AMP second messenger systems. Curr Opin Cell Biol. 1991;3:213–217. - PubMed
-
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

