Antitumor effect of malaria parasite infection in a murine Lewis lung cancer model through induction of innate and adaptive immunity
- PMID: 21931708
- PMCID: PMC3170332
- DOI: 10.1371/journal.pone.0024407
Antitumor effect of malaria parasite infection in a murine Lewis lung cancer model through induction of innate and adaptive immunity
Abstract
Background: Lung cancer is the most common malignancy in humans and its high fatality means that no effective treatment is available. Developing new therapeutic strategies for lung cancer is urgently needed. Malaria has been reported to stimulate host immune responses, which are believed to be efficacious for combating some clinical cancers. This study is aimed to provide evidence that malaria parasite infection is therapeutic for lung cancer.
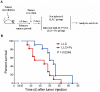
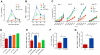
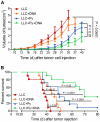
Methodology/principal findings: Antitumor effect of malaria infection was examined in both subcutaneously and intravenously implanted murine Lewis lung cancer (LLC) model. The results showed that malaria infection inhibited LLC growth and metastasis and prolonged the survival of tumor-bearing mice. Histological analysis of tumors from mice infected with malaria revealed that angiogenesis was inhibited, which correlated with increased terminal deoxynucleotidyl transferase-mediated (TUNEL) staining and decreased Ki-67 expression in tumors. Through natural killer (NK) cell cytotoxicity activity, cytokine assays, enzyme-linked immunospot assay, lymphocyte proliferation, and flow cytometry, we demonstrated that malaria infection provided anti-tumor effects by inducing both a potent anti-tumor innate immune response, including the secretion of IFN-γ and TNF-α and the activation of NK cells as well as adaptive anti-tumor immunity with increasing tumor-specific T-cell proliferation and cytolytic activity of CD8(+) T cells. Notably, tumor-bearing mice infected with the parasite developed long-lasting and effective tumor-specific immunity. Consequently, we found that malaria parasite infection could enhance the immune response of lung cancer DNA vaccine pcDNA3.1-hMUC1 and the combination produced a synergistic antitumor effect.
Conclusions/significance: Malaria infection significantly suppresses LLC growth via induction of innate and adaptive antitumor responses in a mouse model. These data suggest that the malaria parasite may provide a novel strategy or therapeutic vaccine vector for anti-lung cancer immune-based therapy.
Conflict of interest statement
Figures



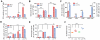
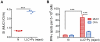
References
-
- Jemal A, Siegel R, Xu J. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Gridelli C, Rossi A, Maione P, Ferrara ML, Castaldo V, et al. Vaccines for the treatment of non-small cell lung cancer: a renewed anticancer strategy. Oncologist. 2009;14:909–920. - PubMed
-
- Kakimi K, Nakajima J, Wada H. Active specific immunotherapy and cell-transfer therapy for the treatment of non-small cell lung cancer. Lung Cancer. 2009;65:1–8. - PubMed
-
- Van den Heuvel MM, Burgers SA, van Zandwijk N. Immunotherapy in non-small-cell lung carcinoma: from inflammation to vaccination. Clin Lung Cancer. 2009;10:99–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

