Determinants of GBP recruitment to Toxoplasma gondii vacuoles and the parasitic factors that control it
- PMID: 21931713
- PMCID: PMC3169597
- DOI: 10.1371/journal.pone.0024434
Determinants of GBP recruitment to Toxoplasma gondii vacuoles and the parasitic factors that control it
Abstract
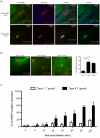
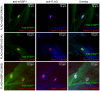
IFN-γ is a major cytokine that mediates resistance against the intracellular parasite Toxoplasma gondii. The p65 guanylate-binding proteins (GBPs) are strongly induced by IFN-γ. We studied the behavior of murine GBP1 (mGBP1) upon infection with T. gondii in vitro and confirmed that IFN-γ-dependent re-localization of mGBP1 to the parasitophorous vacuole (PV) correlates with the virulence type of the parasite. We identified three parasitic factors, ROP16, ROP18, and GRA15 that determine strain-specific accumulation of mGBP1 on the PV. These highly polymorphic proteins are held responsible for a large part of the strain-specific differences in virulence. Therefore, our data suggest that virulence of T. gondii in animals may rely in part on recognition by GBPs. However, phagosomes or vacuoles containing Trypanosoma cruzi did not recruit mGBP1. Co-immunoprecipitation revealed mGBP2, mGBP4, and mGBP5 as binding partners of mGBP1. Indeed, mGBP2 and mGBP5 co-localize with mGBP1 in T. gondii-infected cells. T. gondii thus elicits a cell-autonomous immune response in mice with GBPs involved. Three parasitic virulence factors and unknown IFN-γ-dependent host factors regulate this complex process. Depending on the virulence of the strains involved, numerous GBPs are brought to the PV as part of a large, multimeric structure to combat T. gondii.
Conflict of interest statement
Figures



References
-
- de Veer MJ, Holko M, Frevel M, Walker E, Der S, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. - PubMed
-
- MacMicking JD. IFN-inducible GTPases and immunity to intracellular pathogens. Trends Immunol. 2004;25:601–609. doi: 10.1016/j.it.2004.08.010. - DOI - PubMed
-
- Taylor GA. IRG proteins: key mediators of interferon-regulated host resistance to intracellular pathogens. Cell Microbiol. 2007;9:1099–1107. doi: 10.1111/j.1462-5822.2007.00916.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

