Chondrogenic and gliogenic subpopulations of neural crest play distinct roles during the assembly of epibranchial ganglia
- PMID: 21931719
- PMCID: PMC3170370
- DOI: 10.1371/journal.pone.0024443
Chondrogenic and gliogenic subpopulations of neural crest play distinct roles during the assembly of epibranchial ganglia
Abstract
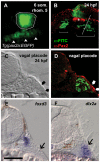
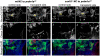
In vertebrates, the sensory neurons of the epibranchial (EB) ganglia transmit somatosensory signals from the periphery to the CNS. These ganglia are formed during embryogenesis by the convergence and condensation of two distinct populations of precursors: placode-derived neuroblasts and neural crest- (NC) derived glial precursors. In addition to the gliogenic crest, chondrogenic NC migrates into the pharyngeal arches, which lie in close proximity to the EB placodes and ganglia. Here, we examine the respective roles of these two distinct NC-derived populations during development of the EB ganglia using zebrafish morphant and mutants that lack one or both of these NC populations. Our analyses of mutant and morphant zebrafish that exhibit deficiencies in chondrogenic NC at early stages reveal a distinct requirement for this NC subpopulation during early EB ganglion assembly and segmentation. Furthermore, restoration of wildtype chondrogenic NC in one of these mutants, prdm1a, is sufficient to restore ganglion formation, indicating a specific requirement of the chondrogenic NC for EB ganglia assembly. By contrast, analysis of the sox10 mutant, which lacks gliogenic NC, reveals that the initial assembly of ganglia is not affected. However, during later stages of development, EB ganglia are dispersed in the sox10 mutant, suggesting that glia are required to maintain normal EB ganglion morphology. These results highlight novel roles for two subpopulations of NC cells in the formation and maintenance of EB ganglia: chondrogenic NC promotes the early-stage formation of the developing EB ganglia while glial NC is required for the late-stage maintenance of ganglion morphology.
Conflict of interest statement
Figures

Similar articles
-
A direct role for Sox10 in specification of neural crest-derived sensory neurons.Development. 2006 Dec;133(23):4619-30. doi: 10.1242/dev.02668. Epub 2006 Oct 25. Development. 2006. PMID: 17065232
-
Development of branchiomeric and lateral line nerves in the axolotl.J Comp Neurol. 1995 May 8;355(3):427-54. doi: 10.1002/cne.903550309. J Comp Neurol. 1995. PMID: 7636024
-
Sox10 is required for the early development of the prospective neural crest in Xenopus embryos.Dev Biol. 2003 Aug 1;260(1):79-96. doi: 10.1016/s0012-1606(03)00247-1. Dev Biol. 2003. PMID: 12885557
-
The cephalic neural crest of amniote vertebrates is composed of a large majority of precursors endowed with neural, melanocytic, chondrogenic and osteogenic potentialities.Cell Cycle. 2010 Jan 15;9(2):238-49. doi: 10.4161/cc.9.2.10491. Epub 2010 Jan 26. Cell Cycle. 2010. PMID: 20037475 Review.
-
Specification of sensory neuron cell fate from the neural crest.Adv Exp Med Biol. 2006;589:170-80. doi: 10.1007/978-0-387-46954-6_10. Adv Exp Med Biol. 2006. PMID: 17076281 Review.
Cited by
-
Skeletogenic fate of zebrafish cranial and trunk neural crest.PLoS One. 2012;7(11):e47394. doi: 10.1371/journal.pone.0047394. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23155370 Free PMC article.
-
The molecular basis of craniofacial placode development.Wiley Interdiscip Rev Dev Biol. 2016 May-Jun;5(3):363-76. doi: 10.1002/wdev.226. Epub 2016 Mar 7. Wiley Interdiscip Rev Dev Biol. 2016. PMID: 26952139 Free PMC article. Review.
-
Preparation of Precisely Oriented Cryosections of Undistorted Drosophila Wing Imaginal Discs for High Resolution Confocal Imaging.Bio Protoc. 2018 Feb 5;8(3):e2725. doi: 10.21769/BioProtoc.2725. Bio Protoc. 2018. PMID: 30167436 Free PMC article.
-
Development of the zebrafish anterior lateral line system is influenced by underlying cranial neural crest.Dev Biol. 2025 Sep;525:102-121. doi: 10.1016/j.ydbio.2025.05.025. Epub 2025 May 29. Dev Biol. 2025. PMID: 40449823
-
prdm1a functions upstream of itga5 in zebrafish craniofacial development.Genesis. 2015 Mar-Apr;53(3-4):270-7. doi: 10.1002/dvg.22850. Epub 2015 Apr 13. Genesis. 2015. PMID: 25810090 Free PMC article.
References
-
- Begbie J, Graham A. Integration between the epibranchial placodes and the hindbrain. Science. 2001;294:595–598. - PubMed
-
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421:189–198. - PubMed
-
- Knight RD, Schilling TF. Cranial neural crest and development of the head skeleton. Adv Exp Med Biol. 2006;589:120–133. - PubMed
-
- Shiau CE, Lwigale PY, Das RM, Wilson SA, Bronner-Fraser M. Robo2-Slit1 dependent cell-cell interactions mediate assembly of the trigeminal ganglion. Nat Neurosci. 2008;11:269–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

