Pre-clinical efficacy and safety of experimental vaccines based on non-replicating vaccinia vectors against yellow fever
- PMID: 21931732
- PMCID: PMC3170363
- DOI: 10.1371/journal.pone.0024505
Pre-clinical efficacy and safety of experimental vaccines based on non-replicating vaccinia vectors against yellow fever
Abstract
Background: Currently existing yellow fever (YF) vaccines are based on the live attenuated yellow fever virus 17D strain (YFV-17D). Although, a good safety profile was historically attributed to the 17D vaccine, serious adverse events have been reported, making the development of a safer, more modern vaccine desirable.
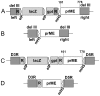
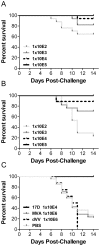
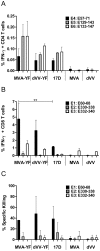
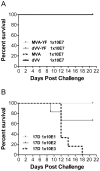
Methodology/principal findings: A gene encoding the precursor of the membrane and envelope (prME) protein of the YFV-17D strain was inserted into the non-replicating modified vaccinia virus Ankara and into the D4R-defective vaccinia virus. Candidate vaccines based on the recombinant vaccinia viruses were assessed for immunogenicity and protection in a mouse model and compared to the commercial YFV-17D vaccine. The recombinant live vaccines induced γ-interferon-secreting CD4- and functionally active CD8-T cells, and conferred full protection against lethal challenge already after a single low immunization dose of 10(5) TCID(50). Surprisingly, pre-existing immunity against wild-type vaccinia virus did not negatively influence protection. Unlike the classical 17D vaccine, the vaccinia virus-based vaccines did not cause mortality following intracerebral administration in mice, demonstrating better safety profiles.
Conclusions/significance: The non-replicating recombinant YF candidate live vaccines induced a broad immune response after single dose administration, were effective even in the presence of a pre-existing immunity against vaccinia virus and demonstrated an excellent safety profile in mice.
Conflict of interest statement
Figures


Similar articles
-
Immunogenicity and Protection After Vaccination With a Modified Vaccinia Virus Ankara-Vectored Yellow Fever Vaccine in the Hamster Model.Front Immunol. 2018 Aug 2;9:1756. doi: 10.3389/fimmu.2018.01756. eCollection 2018. Front Immunol. 2018. PMID: 30116244 Free PMC article.
-
Development of a Bicistronic Yellow Fever Live Attenuated Vaccine with Reduced Neurovirulence and Viscerotropism.Microbiol Spectr. 2022 Oct 26;10(5):e0224622. doi: 10.1128/spectrum.02246-22. Epub 2022 Aug 18. Microbiol Spectr. 2022. PMID: 35980184 Free PMC article.
-
A chimeric yellow fever-Zika virus vaccine candidate fully protects against yellow fever virus infection in mice.Emerg Microbes Infect. 2020 Mar 2;9(1):520-533. doi: 10.1080/22221751.2020.1730709. eCollection 2020. Emerg Microbes Infect. 2020. PMID: 32116148 Free PMC article.
-
The 17D-204 and 17DD yellow fever vaccines: an overview of major similarities and subtle differences.Expert Rev Vaccines. 2018 Jan;17(1):79-90. doi: 10.1080/14760584.2018.1406800. Epub 2017 Nov 27. Expert Rev Vaccines. 2018. PMID: 29172832 Review.
-
Current status and future prospects of yellow fever vaccines.Expert Rev Vaccines. 2015;14(11):1479-92. doi: 10.1586/14760584.2015.1083430. Epub 2015 Sep 14. Expert Rev Vaccines. 2015. PMID: 26366673 Free PMC article. Review.
Cited by
-
Advances and controversies in yellow fever vaccination.Ther Adv Vaccines. 2013 Nov;1(4):144-52. doi: 10.1177/2051013613498954. Ther Adv Vaccines. 2013. PMID: 24757521 Free PMC article. Review.
-
Re-thinking yellow fever vaccines: fighting old foes with new generation vaccines.Hum Vaccin Immunother. 2022 Dec 31;18(1):1895644. doi: 10.1080/21645515.2021.1895644. Epub 2021 May 11. Hum Vaccin Immunother. 2022. PMID: 33974507 Free PMC article. Review.
-
MVA vectors expressing conserved influenza proteins protect mice against lethal challenge with H5N1, H9N2 and H7N1 viruses.PLoS One. 2014 Feb 11;9(2):e88340. doi: 10.1371/journal.pone.0088340. eCollection 2014. PLoS One. 2014. PMID: 24523886 Free PMC article.
-
The Present and Future of Yellow Fever Vaccines.Pharmaceuticals (Basel). 2021 Sep 1;14(9):891. doi: 10.3390/ph14090891. Pharmaceuticals (Basel). 2021. PMID: 34577591 Free PMC article. Review.
-
Selection of recombinant MVA by rescue of the essential D4R gene.Virol J. 2011 Dec 12;8:529. doi: 10.1186/1743-422X-8-529. Virol J. 2011. PMID: 22152060 Free PMC article.
References
-
- WHO. Yellow fever. 2009. Yellow Fever fact sheet No 100 ( http://www.who.int/mediacentre/factsheets/fs100/en/)
-
- Lindenbach BD, Thiel HJ, Rice CM. Flaviviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 1101–1152.
-
- Monath TP. Plotkin SA, Orenstein WA, editors. Yellow Fever. “Vaccines”. 2004. pp. 1095–1176. 4th ed.
-
- Gubler DJ, Kuno G, Markoff L. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 1153–1252.
-
- Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001;1:11–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

