Hydrogen sulfide and neurogenic inflammation in polymicrobial sepsis: involvement of substance P and ERK-NF-κB signaling
- PMID: 21931742
- PMCID: PMC3171449
- DOI: 10.1371/journal.pone.0024535
Hydrogen sulfide and neurogenic inflammation in polymicrobial sepsis: involvement of substance P and ERK-NF-κB signaling
Abstract
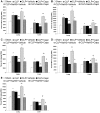
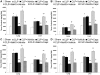
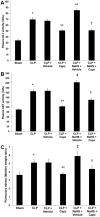
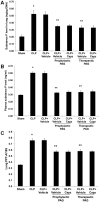
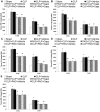
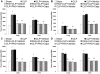
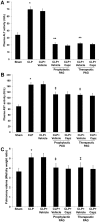
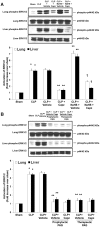
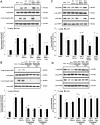
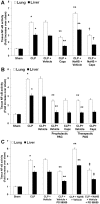
Hydrogen sulfide (H(2)S) has been shown to induce transient receptor potential vanilloid 1 (TRPV1)-mediated neurogenic inflammation in polymicrobial sepsis. However, endogenous neural factors that modulate this event and the molecular mechanism by which this occurs remain unclear. Therefore, this study tested the hypothesis that whether substance P (SP) is one important neural element that implicates in H(2)S-induced neurogenic inflammation in sepsis in a TRPV1-dependent manner, and if so, whether H(2)S regulates this response through activation of the extracellular signal-regulated kinase-nuclear factor-κB (ERK-NF-κB) pathway. Male Swiss mice were subjected to cecal ligation and puncture (CLP)-induced sepsis and treated with TRPV1 antagonist capsazepine 30 minutes before CLP. DL-propargylglycine (PAG), an inhibitor of H(2)S formation, was administrated 1 hour before or 1 hour after sepsis, whereas sodium hydrosulfide (NaHS), an H(2)S donor, was given at the same time as CLP. Capsazepine significantly attenuated H(2)S-induced SP production, inflammatory cytokines, chemokines, and adhesion molecules levels, and protected against lung and liver dysfunction in sepsis. In the absence of H(2)S, capsazepine caused no significant changes to the PAG-mediated attenuation of lung and plasma SP levels, sepsis-associated systemic inflammatory response and multiple organ dysfunction. In addition, capsazepine greatly inhibited phosphorylation of ERK(1/2) and inhibitory κBα, concurrent with suppression of NF-κB activation even in the presence of NaHS. Furthermore, capsazepine had no effect on PAG-mediated abrogation of these levels in sepsis. Taken together, the present findings show that H(2)S regulates TRPV1-mediated neurogenic inflammation in polymicrobial sepsis through enhancement of SP production and activation of the ERK-NF-κB pathway.
Conflict of interest statement
Figures
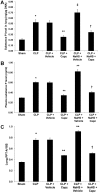
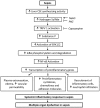
 indicates exogenous administration;
indicates exogenous administration;  indicates inhibition.
indicates inhibition.Similar articles
-
Hydrogen sulfide promotes transient receptor potential vanilloid 1-mediated neurogenic inflammation in polymicrobial sepsis.Crit Care Med. 2010 Feb;38(2):619-28. doi: 10.1097/CCM.0b013e3181c0df00. Crit Care Med. 2010. PMID: 19851090
-
Hydrogen sulfide upregulates cyclooxygenase-2 and prostaglandin E metabolite in sepsis-evoked acute lung injury via transient receptor potential vanilloid type 1 channel activation.J Immunol. 2011 Nov 1;187(9):4778-87. doi: 10.4049/jimmunol.1101559. Epub 2011 Sep 28. J Immunol. 2011. PMID: 21957141
-
Endogenous hydrogen sulfide regulates inflammatory response by activating the ERK pathway in polymicrobial sepsis.J Immunol. 2008 Sep 15;181(6):4320-31. doi: 10.4049/jimmunol.181.6.4320. J Immunol. 2008. PMID: 18768890
-
TRPV1 and SP: key elements for sepsis outcome?Br J Pharmacol. 2013 Dec;170(7):1279-92. doi: 10.1111/bph.12056. Br J Pharmacol. 2013. PMID: 23145480 Free PMC article. Review.
-
Hydrogen gas presents a promising therapeutic strategy for sepsis.Biomed Res Int. 2014;2014:807635. doi: 10.1155/2014/807635. Epub 2014 Apr 16. Biomed Res Int. 2014. PMID: 24829918 Free PMC article. Review.
Cited by
-
Neurokinin-1 Receptor Deficiency Improves Survival in Murine Polymicrobial Sepsis Through Multiple Mechanisms in Aged Mice.Shock. 2019 Jul;52(1):61-66. doi: 10.1097/SHK.0000000000001248. Shock. 2019. PMID: 30095600 Free PMC article.
-
Different Contribution of Redox-Sensitive Transient Receptor Potential Channels to Acetaminophen-Induced Death of Human Hepatoma Cell Line.Front Pharmacol. 2016 Feb 9;7:19. doi: 10.3389/fphar.2016.00019. eCollection 2016. Front Pharmacol. 2016. PMID: 26903865 Free PMC article.
-
High ambient temperature may induce presbyopia via TRPV1 activation.Med Mol Morphol. 2024 Dec;57(4):268-276. doi: 10.1007/s00795-024-00391-2. Epub 2024 Jul 9. Med Mol Morphol. 2024. PMID: 38980406
-
Cannabinoid receptor 2 protects against acute experimental sepsis in mice.Mediators Inflamm. 2013;2013:741303. doi: 10.1155/2013/741303. Epub 2013 May 28. Mediators Inflamm. 2013. PMID: 23781122 Free PMC article.
-
Molecular Biology: Challenges and Opportunities.Curr Issues Mol Biol. 2025 Feb 9;47(2):109. doi: 10.3390/cimb47020109. Curr Issues Mol Biol. 2025. PMID: 39996830 Free PMC article.
References
-
- O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, et al. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. - PubMed
-
- Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

