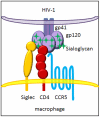
Siglecs facilitate HIV-1 infection of macrophages through adhesion with viral sialic acids
- PMID: 21931755
- PMCID: PMC3169630
- DOI: 10.1371/journal.pone.0024559
Siglecs facilitate HIV-1 infection of macrophages through adhesion with viral sialic acids
Abstract
Background: Human immunodeficiency virus type 1 (HIV-1) infects macrophages effectively, despite relatively low levels of cell surface-expressed CD4. Although HIV-1 infections are defined by viral tropisms according to chemokine receptor usage (R5 and X4), variations in infection are common within both R5- and X4-tropic viruses, indicating additional factors may contribute to viral tropism.
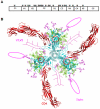
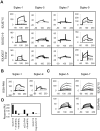
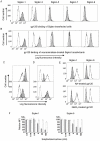
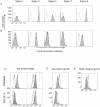
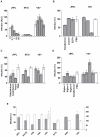
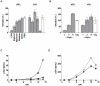
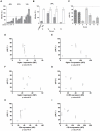
Methodology and principal findings: Using both solution and cell surface binding experiments, we showed that R5- and X4-tropic HIV-1 gp120 proteins recognized a family of I-type lectin receptors, the Sialic acid-binding immunoglobulin-like lectins (Siglec). The recognition was through envelope-associated sialic acids that promoted viral adhesion to macrophages. The sialic acid-mediated viral-host interaction facilitated both R5-tropic pseudovirus and HIV-1(BaL) infection of macrophages. The high affinity Siglec-1 contributed the most to HIV-1 infection and the variation in Siglec-1 expression on primary macrophages from different donors was associated statistically with sialic acid-facilitated viral infection. Furthermore, envelope-associated sialoglycan variations on various strains of R5-tropic viruses also affected infection.
Conclusions and significance of the findings: Our study showed that sialic acids on the viral envelope facilitated HIV-1 infection of macrophages through interacting with Siglec receptors, and the expression of Siglec-1 correlated with viral sialic acid-mediated host attachment. This glycan-mediated viral adhesion underscores the importance of viral sialic acids in HIV infection and pathogenesis, and suggests a novel class of antiviral compounds targeting Siglec receptors.
Conflict of interest statement
Figures
Similar articles
-
HIV-1 R5 Macrophage-Tropic Envelope Glycoprotein Trimers Bind CD4 with High Affinity, while the CD4 Binding Site on Non-macrophage-tropic, T-Tropic R5 Envelopes Is Occluded.J Virol. 2018 Jan 2;92(2):e00841-17. doi: 10.1128/JVI.00841-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29118121 Free PMC article.
-
Effect of cytokines on Siglec-1 and HIV-1 entry in monocyte-derived macrophages: the importance of HIV-1 envelope V1V2 region.J Leukoc Biol. 2016 Jun;99(6):1089-106. doi: 10.1189/jlb.2A0815-361R. Epub 2015 Dec 14. J Leukoc Biol. 2016. PMID: 26667473 Free PMC article.
-
Sialic acid-binding Ig-like lectin-7 interacts with HIV-1 gp120 and facilitates infection of CD4pos T cells and macrophages.Retrovirology. 2013 Dec 13;10:154. doi: 10.1186/1742-4690-10-154. Retrovirology. 2013. PMID: 24330394 Free PMC article.
-
HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV.J Transl Med. 2011 Jan 27;9 Suppl 1(Suppl 1):S2. doi: 10.1186/1479-5876-9-S1-S2. J Transl Med. 2011. PMID: 21284901 Free PMC article. Review.
-
Siglecs: A journey through the evolution of sialic acid-binding immunoglobulin-type lectins.Dev Comp Immunol. 2018 Sep;86:219-231. doi: 10.1016/j.dci.2018.05.008. Epub 2018 May 8. Dev Comp Immunol. 2018. PMID: 29751010 Review.
Cited by
-
Characterising plasmacytoid and myeloid AXL+ SIGLEC-6+ dendritic cell functions and their interactions with HIV.PLoS Pathog. 2024 Jun 26;20(6):e1012351. doi: 10.1371/journal.ppat.1012351. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38924030 Free PMC article.
-
The role of Siglec-1 and SR-BI interaction in the phagocytosis of oxidized low density lipoprotein by macrophages.PLoS One. 2013;8(3):e58831. doi: 10.1371/journal.pone.0058831. Epub 2013 Mar 8. PLoS One. 2013. PMID: 23520536 Free PMC article.
-
The α2,3-sialyltransferase encoded by myxoma virus is a virulence factor that contributes to immunosuppression.PLoS One. 2015 Feb 23;10(2):e0118806. doi: 10.1371/journal.pone.0118806. eCollection 2015. PLoS One. 2015. PMID: 25705900 Free PMC article.
-
Identification of lipophilic ligands of Siglec5 and -14 that modulate innate immune responses.J Biol Chem. 2019 Nov 8;294(45):16776-16788. doi: 10.1074/jbc.RA119.009835. Epub 2019 Sep 24. J Biol Chem. 2019. PMID: 31551352 Free PMC article.
-
The role of Siglec-1 in HIV-1/macrophage interaction.Macrophage (Houst). 2016;3:e1435. Epub 2016 Sep 17. Macrophage (Houst). 2016. PMID: 28286869 Free PMC article.
References
-
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. - PubMed
-
- Wu L, Gerard NP, Wyatt R, Choe H, Parolin C, et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. - PubMed
-
- Montaner LJ, Crowe SM, Aquaro S, Perno CF, Stevenson M, et al. Advances in macrophage and dendritic cell biology in HIV-1 infection stress key understudied areas in infection, pathogenesis, and analysis of viral reservoirs. J Leukoc Biol. 2006;80:961–964. - PubMed
-
- Kazmierczak K, Potash MJ. Host and virus strain dependence in activation of human macrophages by human immunodeficiency virus type 1. J Neurovirol. 2007;13:452–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

