Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus
- PMID: 21931758
- PMCID: PMC3172233
- DOI: 10.1371/journal.pone.0024566
Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus
Abstract
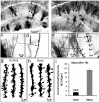
Hippocampal injury-associated learning and memory deficits are frequent hallmarks of brain trauma and are the most enduring and devastating consequences following traumatic brain injury (TBI). Several reports, including our recent paper, showed that TBI brought on by a moderate level of controlled cortical impact (CCI) induces immature newborn neuron death in the hippocampal dentate gyrus. In contrast, the majority of mature neurons are spared. Less research has been focused on these spared neurons, which may also be injured or compromised by TBI. Here we examined the dendrite morphologies, dendritic spines, and synaptic structures using a genetic approach in combination with immunohistochemistry and Golgi staining. We found that although most of the mature granular neurons were spared following TBI at a moderate level of impact, they exhibited dramatic dendritic beading and fragmentation, decreased number of dendritic branches, and a lower density of dendritic spines, particularly the mushroom-shaped mature spines. Further studies showed that the density of synapses in the molecular layer of the hippocampal dentate gyrus was significantly reduced. The electrophysiological activity of neurons was impaired as well. These results indicate that TBI not only induces cell death in immature granular neurons, it also causes significant dendritic and synaptic degeneration in pathohistology. TBI also impairs the function of the spared mature granular neurons in the hippocampal dentate gyrus. These observations point to a potential anatomic substrate to explain, in part, the development of posttraumatic memory deficits. They also indicate that dendritic damage in the hippocampal dentate gyrus may serve as a therapeutic target following TBI.
Conflict of interest statement
Figures





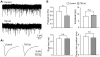
Similar articles
-
Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury.J Neurosci Res. 2008 Aug 1;86(10):2258-70. doi: 10.1002/jnr.21677. J Neurosci Res. 2008. PMID: 18381764 Free PMC article.
-
Impaired dendritic development and synaptic formation of postnatal-born dentate gyrus granular neurons in the absence of brain-derived neurotrophic factor signaling.Exp Neurol. 2009 Jan;215(1):178-90. doi: 10.1016/j.expneurol.2008.10.009. Epub 2008 Oct 30. Exp Neurol. 2009. PMID: 19014937
-
Dendritic morphological development of traumatic brain injury-induced new neurons in the dentate gyrus is important for post-injury cognitive recovery and is regulated by Notch1.Exp Neurol. 2024 Dec;382:114963. doi: 10.1016/j.expneurol.2024.114963. Epub 2024 Sep 18. Exp Neurol. 2024. PMID: 39303845
-
Remodeling dendritic spines for treatment of traumatic brain injury.Neural Regen Res. 2019 Sep;14(9):1477-1480. doi: 10.4103/1673-5374.255957. Neural Regen Res. 2019. PMID: 31089035 Free PMC article. Review.
-
The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies).Prog Brain Res. 2007;163:3-22. doi: 10.1016/S0079-6123(07)63001-5. Prog Brain Res. 2007. PMID: 17765709 Free PMC article. Review.
Cited by
-
Neuropathophysiological Mechanisms and Treatment Strategies for Post-traumatic Epilepsy.Front Mol Neurosci. 2021 Feb 23;14:612073. doi: 10.3389/fnmol.2021.612073. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33708071 Free PMC article. Review.
-
Traumatic Brain Injury Impairs Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptor Complex Formation and Alters Synaptic Vesicle Distribution in the Hippocampus.J Neurotrauma. 2016 Jan 1;33(1):113-21. doi: 10.1089/neu.2014.3839. Epub 2015 Aug 27. J Neurotrauma. 2016. PMID: 25923735 Free PMC article.
-
Traumatic Axonal Injury: Mechanisms and Translational Opportunities.Trends Neurosci. 2016 May;39(5):311-324. doi: 10.1016/j.tins.2016.03.002. Epub 2016 Mar 31. Trends Neurosci. 2016. PMID: 27040729 Free PMC article. Review.
-
Clonidine Protects Against Neurotoxicity Induced by Sevoflurane Through NF-κB Signaling Inhibition and Proinflammatory Cytokine Release in Rats.J Mol Neurosci. 2018 Aug;65(4):507-513. doi: 10.1007/s12031-018-1117-z. Epub 2018 Aug 2. J Mol Neurosci. 2018. PMID: 30073555
-
Paclitaxel Reduces Brain Injury from Repeated Head Trauma in Mice.J Alzheimers Dis. 2019;67(3):859-874. doi: 10.3233/JAD-180871. J Alzheimers Dis. 2019. PMID: 30664506 Free PMC article.
References
-
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, et al. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252–265. - PubMed
-
- Saatman KE, Feeko KJ, Pape RL, Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J Neurotrauma. 2006;23:1241–1253. - PubMed
-
- Adelson PD, Dixon CE, Robichaud P, Kochanek PM. Motor and cognitive functional deficits following diffuse traumatic brain injury in the immature rat. J Neurotrauma. 1997;14:99–108. - PubMed
-
- Lighthall JW. Controlled cortical impact: a new experimental brain injury model. J Neurotrauma. 1988;5:1–15. - PubMed
-
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, et al. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22:42–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

