Hepatocyte growth factor (HGF) inhibits collagen I and IV synthesis in hepatic stellate cells by miRNA-29 induction
- PMID: 21931759
- PMCID: PMC3170366
- DOI: 10.1371/journal.pone.0024568
Hepatocyte growth factor (HGF) inhibits collagen I and IV synthesis in hepatic stellate cells by miRNA-29 induction
Abstract
Background: In chronic liver disease, hepatic stellate cells (HSC) transdifferentiate into myofibroblasts, promoting extracellular matrix (ECM) synthesis and deposition. Stimulation of HSC by transforming growth factor-β (TGF-β) is a crucial event in liver fibrogenesis due to its impact on myofibroblastic transition and ECM induction. In contrast, hepatocyte growth factor (HGF), exerts antifibrotic activities. Recently, miR-29 has been reported to be involved in ECM synthesis. We therefore studied the influence of HGF and TGF-β on the miR-29 collagen axis in HSC.
Methodology: HSC, isolated from rats, were characterized for HGF and Met receptor expression by Real-Time PCR and Western blotting during culture induced myofibroblastic transition. Then, the levels of TGF-β, HGF, collagen-I and -IV mRNA, in addition to miR-29a and miR-29b were determined after HGF and TGF-β stimulation of HSC or after experimental fibrosis induced by bile-duct obstruction in rats. The interaction of miR-29 with 3'-untranslated mRNA regions (UTR) was analyzed by reporter assays. The repressive effect of miR-29 on collagen synthesis was studied in HSC treated with miR-29-mimicks by Real-Time PCR and immunoblotting.
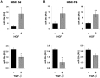
Principal findings: The 3'-UTR of the collagen-1 and -4 subtypes were identified to bind miR-29. Hence, miR-29a/b overexpression in HSC resulted in a marked reduction of collagen-I and -IV synthesis. Conversely, a decrease in miR-29 levels is observed during collagen accumulation upon experimental fibrosis, in vivo, and after TGF-β stimulation of HSC, in vitro. Finally, we show that during myofibroblastic transition and TGF-β exposure the HGF-receptor, Met, is upregulated in HSC. Thus, whereas TGF-β stimulation leads to a reduction in miR-29 expression and de-repression of collagen synthesis, stimulation with HGF was definitely associated with highly elevated miR-29 levels and markedly repressed collagen-I and -IV synthesis.
Conclusions: Upregulation of miRNA-29 by HGF and downregulation by TGF-β take part in the anti- or profibrogenic response of HSC, respectively.
Conflict of interest statement
Figures

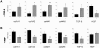

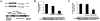




References
-
- Schuppan D, Krebs A, Bauer M, Hahn EG. Hepatitis C and liver fibrosis. Cell Death Differ. 2003;10(Suppl 1):S59–67. - PubMed
-
- Gressner OA, Rizk MS, Kovalenko E, Weiskirchen R, Gressner AM. Changing the pathogenetic roadmap of liver fibrosis? Where did it start; where will it go? J Gastroenterol Hepatol. 2008;23:1024–1035. - PubMed
-
- Geerts A, Schuppan D, Lazeroms S, De Zanger R, Wisse E. Collagen type I and III occur together in hybrid fibrils in the space of Disse of normal rat liver. Hepatology. 1990;12:233–241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

