Suppression of breast tumor growth and metastasis by an engineered transcription factor
- PMID: 21931769
- PMCID: PMC3172243
- DOI: 10.1371/journal.pone.0024595
Suppression of breast tumor growth and metastasis by an engineered transcription factor
Abstract
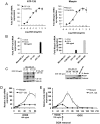
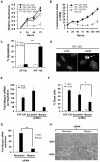
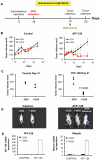
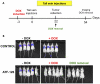
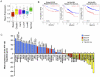
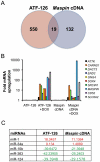
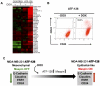
Maspin is a tumor and metastasis suppressor playing an essential role as gatekeeper of tumor progression. It is highly expressed in epithelial cells but is silenced in the onset of metastatic disease by epigenetic mechanisms. Reprogramming of Maspin epigenetic silencing offers a therapeutic potential to lock metastatic progression. Herein we have investigated the ability of the Artificial Transcription Factor 126 (ATF-126) designed to upregulate the Maspin promoter to inhibit tumor progression in pre-established breast tumors in immunodeficient mice. ATF-126 was transduced in the aggressive, mesenchymal-like and triple negative breast cancer line, MDA-MB-231. Induction of ATF expression in vivo by Doxycycline resulted in 50% reduction in tumor growth and totally abolished tumor cell colonization. Genome-wide transcriptional profiles of ATF-induced cells revealed a gene signature that was found over-represented in estrogen receptor positive (ER+) "Normal-like" intrinsic subtype of breast cancer and in poorly aggressive, ER+ luminal A breast cancer cell lines. The comparison transcriptional profiles of ATF-126 and Maspin cDNA defined an overlapping 19-gene signature, comprising novel targets downstream the Maspin signaling cascade. Our data suggest that Maspin up-regulates downstream tumor and metastasis suppressor genes that are silenced in breast cancers, and are normally expressed in the neural system, including CARNS1, SLC8A2 and DACT3. In addition, ATF-126 and Maspin cDNA induction led to the re-activation of tumor suppressive miRNAs also expressed in neural cells, such as miR-1 and miR-34, and to the down-regulation of potential oncogenic miRNAs, such as miR-10b, miR-124, and miR-363. As expected from its over-representation in ER+ tumors, the ATF-126-gene signature predicted favorable prognosis for breast cancer patients. Our results describe for the first time an ATF able to reduce tumor growth and metastatic colonization by epigenetic reactivation of a dormant, normal-like, and more differentiated gene program.
Conflict of interest statement
Figures
Similar articles
-
Re-activation of a dormant tumor suppressor gene maspin by designed transcription factors.Oncogene. 2007 Apr 26;26(19):2791-8. doi: 10.1038/sj.onc.1210072. Epub 2006 Oct 23. Oncogene. 2007. PMID: 17057734
-
Reactivation of MASPIN in non-small cell lung carcinoma (NSCLC) cells by artificial transcription factors (ATFs).Epigenetics. 2011 Feb;6(2):224-35. doi: 10.4161/epi.6.2.13700. Epub 2011 Feb 1. Epigenetics. 2011. PMID: 20948306 Free PMC article.
-
Reprogramming epigenetic silencing: artificial transcription factors synergize with chromatin remodeling drugs to reactivate the tumor suppressor mammary serine protease inhibitor.Mol Cancer Ther. 2008 May;7(5):1080-90. doi: 10.1158/1535-7163.MCT-07-0526. Mol Cancer Ther. 2008. PMID: 18483297 Free PMC article.
-
Modeling human breast cancer metastasis in mice: maspin as a paradigm.Histol Histopathol. 2003 Jan;18(1):201-6. doi: 10.14670/HH-18.201. Histol Histopathol. 2003. PMID: 12507299 Review.
-
Multiple functions of maspin in tumor progression and mouse development.Front Biosci. 2004 Sep 1;9:2218-26. doi: 10.2741/1392. Front Biosci. 2004. PMID: 15353283 Review.
Cited by
-
Prolonged re-expression of the hypermethylated gene EPB41L3 using artificial transcription factors and epigenetic drugs.Epigenetics. 2015;10(5):384-96. doi: 10.1080/15592294.2015.1034415. Epub 2015 Apr 1. Epigenetics. 2015. PMID: 25830725 Free PMC article.
-
Novel role of Engrailed 1 as a prosurvival transcription factor in basal-like breast cancer and engineering of interference peptides block its oncogenic function.Oncogene. 2014 Sep 25;33(39):4767-77. doi: 10.1038/onc.2013.422. Epub 2013 Oct 21. Oncogene. 2014. PMID: 24141779 Free PMC article.
-
Expression and localization of maspin in cervical cancer and its role in tumor progression and lymphangiogenesis.Arch Gynecol Obstet. 2014 Feb;289(2):373-82. doi: 10.1007/s00404-013-2988-4. Arch Gynecol Obstet. 2014. PMID: 23959090 Free PMC article.
-
Role of microRNA-1 in human cancer and its therapeutic potentials.Biomed Res Int. 2014;2014:428371. doi: 10.1155/2014/428371. Epub 2014 May 18. Biomed Res Int. 2014. PMID: 24949449 Free PMC article. Review.
-
Writing and rewriting the epigenetic code of cancer cells: from engineered proteins to small molecules.Mol Pharmacol. 2013 Mar;83(3):563-76. doi: 10.1124/mol.112.080697. Epub 2012 Nov 13. Mol Pharmacol. 2013. PMID: 23150486 Free PMC article. Review.
References
-
- Sager R, Sheng S, Pemberton P, Hendrix MJ. Maspin. A tumor suppressing serpin. Adv Exp Med Biol. 1997;425:77–88. - PubMed
-
- Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–199. - PubMed
-
- Nakagawa M, Katakura H, Adachi M, Takenaka K, Yanagihara K, et al. Maspin expression and its clinical significance in non-small cell lung cancer. Ann Surg Oncol. 2006;13:1517–1523. - PubMed
-
- Machtens S, Serth J, Bokemeyer C, Bathke W, Minssen A, et al. Expression of the p53 and Maspin protein in primary prostate cancer: correlation with clinical features. Int J Cancer. 2001;95:337–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

