Structure of apo- and monometalated forms of NDM-1--a highly potent carbapenem-hydrolyzing metallo-β-lactamase
- PMID: 21931780
- PMCID: PMC3169612
- DOI: 10.1371/journal.pone.0024621
Structure of apo- and monometalated forms of NDM-1--a highly potent carbapenem-hydrolyzing metallo-β-lactamase
Abstract
The New Delhi Metallo-β-lactamase (NDM-1) gene makes multiple pathogenic microorganisms resistant to all known β-lactam antibiotics. The rapid emergence of NDM-1 has been linked to mobile plasmids that move between different strains resulting in world-wide dissemination. Biochemical studies revealed that NDM-1 is capable of efficiently hydrolyzing a wide range of β-lactams, including many carbapenems considered as "last resort" antibiotics. The crystal structures of metal-free apo- and monozinc forms of NDM-1 presented here revealed an enlarged and flexible active site of class B1 metallo-β-lactamase. This site is capable of accommodating many β-lactam substrates by having many of the catalytic residues on flexible loops, which explains the observed extended spectrum activity of this zinc dependent β-lactamase. Indeed, five loops contribute "keg" residues in the active site including side chains involved in metal binding. Loop 1 in particular, shows conformational flexibility, apparently related to the acceptance and positioning of substrates for cleavage by a zinc-activated water molecule.
Conflict of interest statement
Figures
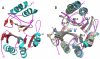
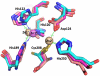
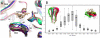

Similar articles
-
New Delhi Metallo-beta lactamase (NDM-1) in Enterobacteriaceae: treatment options with carbapenems compromised.J Assoc Physicians India. 2010 Mar;58:147-9. J Assoc Physicians India. 2010. PMID: 20848811
-
Characterization of purified New Delhi metallo-β-lactamase-1.Biochemistry. 2011 Nov 22;50(46):10102-13. doi: 10.1021/bi201449r. Epub 2011 Nov 1. Biochemistry. 2011. PMID: 22029287
-
New Delhi metallo-β-lactamase: structural insights into β-lactam recognition and inhibition.J Am Chem Soc. 2012 Jul 18;134(28):11362-5. doi: 10.1021/ja303579d. Epub 2012 Jul 5. J Am Chem Soc. 2012. PMID: 22713171
-
Structure, Genetics and Worldwide Spread of New Delhi Metallo-β-lactamase (NDM): a threat to public health.BMC Microbiol. 2017 Apr 27;17(1):101. doi: 10.1186/s12866-017-1012-8. BMC Microbiol. 2017. PMID: 28449650 Free PMC article. Review.
-
Structural and functional insight of New Delhi Metallo β-lactamase-1 variants.Future Med Chem. 2018 Jan;10(2):221-229. doi: 10.4155/fmc-2017-0143. Epub 2017 Dec 5. Future Med Chem. 2018. PMID: 29202600 Review.
Cited by
-
Oligopeptides as full-length New Delhi metallo-β-lactamase-1 (NDM-1) inhibitors.PLoS One. 2017 May 23;12(5):e0177293. doi: 10.1371/journal.pone.0177293. eCollection 2017. PLoS One. 2017. PMID: 28542279 Free PMC article.
-
Biochemical analysis of metallo-β-lactamase NDM-3 from a multidrug-resistant Escherichia coli strain isolated in Japan.Antimicrob Agents Chemother. 2014 Jun;58(6):3538-40. doi: 10.1128/AAC.02793-13. Epub 2014 Mar 31. Antimicrob Agents Chemother. 2014. PMID: 24687501 Free PMC article.
-
Faropenem reacts with serine and metallo-β-lactamases to give multiple products.Eur J Med Chem. 2021 Apr 5;215:113257. doi: 10.1016/j.ejmech.2021.113257. Epub 2021 Feb 9. Eur J Med Chem. 2021. PMID: 33618159 Free PMC article.
-
A quantum mechanics/molecular mechanics study on the hydrolysis mechanism of New Delhi metallo-β-lactamase-1.J Comput Aided Mol Des. 2013 Mar;27(3):247-56. doi: 10.1007/s10822-012-9630-6. Epub 2013 Mar 2. J Comput Aided Mol Des. 2013. PMID: 23456591
-
Biochemical characteristics of New Delhi metallo-β-lactamase-1 show unexpected difference to other MBLs.PLoS One. 2013 Apr 12;8(4):e61914. doi: 10.1371/journal.pone.0061914. Print 2013. PLoS One. 2013. PMID: 23593503 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

