Acquisition of chemoresistance in gliomas is associated with increased mitochondrial coupling and decreased ROS production
- PMID: 21931801
- PMCID: PMC3170372
- DOI: 10.1371/journal.pone.0024665
Acquisition of chemoresistance in gliomas is associated with increased mitochondrial coupling and decreased ROS production
Abstract
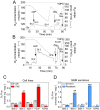
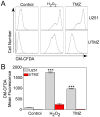
Temozolomide (TMZ) is an alkylating agent used for treating gliomas. Chemoresistance is a severe limitation to TMZ therapy; there is a critical need to understand the underlying mechanisms that determine tumor response to TMZ. We recently reported that chemoresistance to TMZ is related to a remodeling of the entire electron transport chain, with significant increases in the activity of complexes II/III and cytochrome c oxidase (CcO). Moreover, pharmacologic and genetic manipulation of CcO reverses chemoresistance. Therefore, to test the hypothesis that TMZ-resistance arises from tighter mitochondrial coupling and decreased production of reactive oxygen species (ROS), we have assessed mitochondrial function in TMZ-sensitive and -resistant glioma cells, and in TMZ-resistant glioblastoma multiform (GBM) xenograft lines (xenolines). Maximum ADP-stimulated (state 3) rates of mitochondrial oxygen consumption were greater in TMZ-resistant cells and xenolines, and basal respiration (state 2), proton leak (state 4), and mitochondrial ROS production were significantly lower in TMZ-resistant cells. Furthermore, TMZ-resistant cells consumed less glucose and produced less lactic acid. Chemoresistant cells were insensitive to the oxidative stress induced by TMZ and hydrogen peroxide challenges, but treatment with the oxidant L-buthionine-S,R-sulfoximine increased TMZ-dependent ROS generation and reversed chemoresistance. Importantly, treatment with the antioxidant N-acetyl-cysteine inhibited TMZ-dependent ROS generation in chemosensitive cells, preventing TMZ toxicity. Finally, we found that mitochondrial DNA-depleted cells (ρ°) were resistant to TMZ and had lower intracellular ROS levels after TMZ exposure compared with parental cells. Repopulation of ρ° cells with mitochondria restored ROS production and sensitivity to TMZ. Taken together, our results indicate that chemoresistance to TMZ is linked to tighter mitochondrial coupling and low ROS production, and suggest a novel mitochondrial ROS-dependent mechanism underlying TMZ-chemoresistance in glioma. Thus, perturbation of mitochondrial functions and changes in redox status might constitute a novel strategy for sensitizing glioma cells to therapeutic approaches.
Conflict of interest statement
Figures
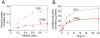





References
-
- Saybasili H, Yuksel M, Haklar G, Yalcin AS. Effect of mitochondrial electron transport chain inhibitors on superoxide radical generation in rat hippocampal and striatal slices. Antioxid Redox Signal. 2001;3:1099–1104. - PubMed
-
- Staniek K, Gille L, Kozlov AV, Nohl H. Mitochondrial superoxide radical formation is controlled by electron bifurcation to the high and low potential pathways. Free Radic Res. 2002;36:381–387. - PubMed
-
- Richter C, Gogvadze V, Laffranchi R, Schlapbach R, Schweizer M, et al. Oxidants in mitochondria: from physiology to diseases. Biochim Biophys Acta. 1995;1271:67–74. - PubMed
-
- Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

