Maintaining vaccine delivery following the introduction of the rotavirus and pneumococcal vaccines in Thailand
- PMID: 21931805
- PMCID: PMC3172252
- DOI: 10.1371/journal.pone.0024673
Maintaining vaccine delivery following the introduction of the rotavirus and pneumococcal vaccines in Thailand
Abstract
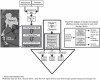
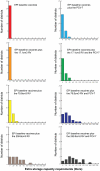
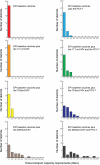
Although the substantial burdens of rotavirus and pneumococcal disease have motivated many countries to consider introducing the rotavirus vaccine (RV) and heptavalent pneumococcal conjugate vaccine (PCV-7) to their National Immunization Programs (EPIs), these new vaccines could affect the countries' vaccine supply chains (i.e., the series of steps required to get a vaccine from their manufacturers to patients). We developed detailed computational models of the Trang Province, Thailand, vaccine supply chain to simulate introducing various RV and PCV-7 vaccine presentations and their combinations. Our results showed that the volumes of these new vaccines in addition to current routine vaccines could meet and even exceed (1) the refrigerator space at the provincial district and sub-district levels and (2) the transport cold space at district and sub-district levels preventing other vaccines from being available to patients who arrive to be immunized. Besides the smallest RV presentation (17.1 cm³/dose), all other vaccine introduction scenarios required added storage capacity at the provincial level (range: 20 L-1151 L per month) for the three largest formulations, and district level (range: 1 L-124 L per month) across all introduction scenarios. Similarly, with the exception of the two smallest RV presentation (17.1 cm³/dose), added transport capacity was required at both district and sub-district levels. Added transport capacity required across introduction scenarios from the provincial to district levels ranged from 1 L-187 L, and district to sub-district levels ranged from 1 L-13 L per shipment. Finally, only the smallest RV vaccine presentation (17.1 cm³/dose) had no appreciable effect on vaccine availability at sub-districts. All other RV and PCV-7 vaccines were too large for the current supply chain to handle without modifications such as increasing storage or transport capacity. Introducing these new vaccines to Thailand could have dynamic effects on the availability of all vaccines that may not be initially apparent to decision-makers.
Conflict of interest statement
Figures
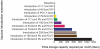
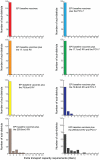
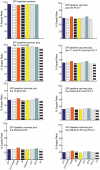
References
-
- de Oliveira LH, Danovaro-Holliday MC, Matus CR, Andrus JK. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert Rev Vaccines. 2008;7:345–353. - PubMed
-
- PATH. The PATH Rotavirus Vaccine Program: Summary report. Seattle, Washington: PATH; 2009. pp. 1–16.
-
- WHO. Mortality country fact sheet: Thailand. 2006. Bulletin of the World Health Organization.
-
- IBM. 2010. C and C++ Compilers.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

