Dissimilarity in the folding of human cytosolic creatine kinase isoenzymes
- PMID: 21931810
- PMCID: PMC3170377
- DOI: 10.1371/journal.pone.0024681
Dissimilarity in the folding of human cytosolic creatine kinase isoenzymes
Abstract
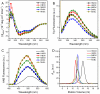
Creatine kinase (CK, EC 2.7.3.2) plays a key role in the energy homeostasis of excitable cells. The cytosolic human CK isoenzymes exist as homodimers (HMCK and HBCK) or a heterodimer (MBCK) formed by the muscle CK subunit (M) and/or brain CK subunit (B) with highly conserved three-dimensional structures composed of a small N-terminal domain (NTD) and a large C-terminal domain (CTD). The isoforms of CK provide a novel system to investigate the sequence/structural determinants of multimeric/multidomain protein folding. In this research, the role of NTD and CTD as well as the domain interactions in CK folding was investigated by comparing the equilibrium and kinetic folding parameters of HMCK, HBCK, MBCK and two domain-swapped chimeric forms (BnMc and MnBc). Spectroscopic results indicated that the five proteins had distinct structural features depending on the domain organizations. MBCK BnMc had the smallest CD signals and the lowest stability against guanidine chloride-induced denaturation. During the biphasic kinetic refolding, three proteins (HMCK, BnMc and MnBc), which contained either the NTD or CTD of the M subunit and similar microenvironments of the Trp fluorophores, refolded about 10-fold faster than HBCK for both the fast and slow phase. The fast folding of these three proteins led to an accumulation of the aggregation-prone intermediate and slowed down the reactivation rate thereby during the kinetic refolding. Our results suggested that the intra- and inter-subunit domain interactions modified the behavior of kinetic refolding. The alternation of domain interactions based on isoenzymes also provides a valuable strategy to improve the properties of multidomain enzymes in biotechnology.
Conflict of interest statement
Figures




Similar articles
-
Relationship between kinetic and equilibrium folding intermediates of creatine kinase.Biochem Biophys Res Commun. 2001 Jul 27;285(4):857-62. doi: 10.1006/bbrc.2001.5248. Biochem Biophys Res Commun. 2001. PMID: 11467829
-
Catalysis of creatine kinase refolding by protein disulfide isomerase involves disulfide cross-link and dimer to tetramer switch.J Biol Chem. 2005 Apr 8;280(14):13470-6. doi: 10.1074/jbc.M413882200. Epub 2005 Feb 5. J Biol Chem. 2005. PMID: 15695804
-
Effects of aspartate on rabbit muscle creatine kinase and the salt induced molten globule state.Int J Biochem Cell Biol. 2002 Aug;34(8):970-82. doi: 10.1016/s1357-2725(02)00018-3. Int J Biochem Cell Biol. 2002. PMID: 12007635
-
[Creatine kinase isoenzymes--characterization and functions in cell].Postepy Biochem. 2008;54(3):274-83. Postepy Biochem. 2008. PMID: 19112826 Review. Polish.
-
Some new aspects of creatine kinase (CK): compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiology.Biofactors. 1998;8(3-4):229-34. doi: 10.1002/biof.5520080310. Biofactors. 1998. PMID: 9914824 Review.
Cited by
-
A single residue substitution accounts for the significant difference in thermostability between two isoforms of human cytosolic creatine kinase.Sci Rep. 2016 Feb 16;6:21191. doi: 10.1038/srep21191. Sci Rep. 2016. PMID: 26879258 Free PMC article.
-
Evaluation of antifatigue and antioxidant activities of the marine microalgae Isochrysis galbana in mice.Food Sci Biotechnol. 2019 Nov 27;29(4):549-557. doi: 10.1007/s10068-019-00694-6. eCollection 2020 Apr. Food Sci Biotechnol. 2019. PMID: 32296566 Free PMC article.
-
Model of the Ankyrin and SOCS Box Protein, ASB9, E3 Ligase Reveals a Mechanism for Dynamic Ubiquitin Transfer.Structure. 2016 Aug 2;24(8):1248-1256. doi: 10.1016/j.str.2016.05.016. Epub 2016 Jul 7. Structure. 2016. PMID: 27396830 Free PMC article.
-
Effect of SNPs on creatine kinase structure and function: identifying potential molecular mechanisms for possible creatine kinase deficiency diseases.PLoS One. 2012;7(9):e45949. doi: 10.1371/journal.pone.0045949. Epub 2012 Sep 25. PLoS One. 2012. PMID: 23049898 Free PMC article.
-
Molecular Characterization, Expression Profile, and A 21-bp Indel within the ASB9 Gene and Its Associations with Chicken Production Traits.Genes (Basel). 2023 Jan 28;14(2):339. doi: 10.3390/genes14020339. Genes (Basel). 2023. PMID: 36833266 Free PMC article.
References
-
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isozymes in tissues with high and fluctuating energy demands: The "phosphocreatine circuit" for cellular energy homeostasis. Biochem J. 1992;281:21–40. - PMC - PubMed
-
- Chen Z, Zhao TJ, Li J, Gao YS, Meng FG, et al. Slow skeletal muscle myosin-binding protein-C (MyBPC1) mediates recruitment of muscle-type creatine kinase (CK) to myosin. Biochem J. 2011;436:437–445. - PubMed
-
- Zhao TJ, Yan YB, Liu Y, Zhou HM. The generation of the oxidized form of creatine kinase is a negative regulation on muscle creatine kinase. J Biol Chem. 2007;282:12022–12029. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

