Lumican expression in diaphragm induced by mechanical ventilation
- PMID: 21931815
- PMCID: PMC3170381
- DOI: 10.1371/journal.pone.0024692
Lumican expression in diaphragm induced by mechanical ventilation
Abstract
Background: Diaphragmatic dysfunction found in the patients with acute lung injury required prolonged mechanical ventilation. Mechanical ventilation can induce production of inflammatory cytokines and excess deposition of extracellular matrix proteins via up-regulation of transforming growth factor (TGF)-β1. Lumican is known to participate in TGF-β1 signaling during wound healing. The mechanisms regulating interactions between mechanical ventilation and diaphragmatic injury are unclear. We hypothesized that diaphragmatic damage by short duration of mechanical stretch caused up-regulation of lumican that modulated TGF-β1 signaling.
Methods: Male C57BL/6 mice, either wild-type or lumican-null, aged 3 months, weighing between 25 and 30 g, were exposed to normal tidal volume (10 ml/kg) or high tidal volume (30 ml/kg) mechanical ventilation with room air for 2 to 8 hours. Nonventilated mice served as control groups.
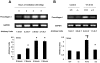
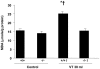
Results: High tidal volume mechanical ventilation induced interfibrillar disassembly of diaphragmatic collagen fiber, lumican activation, type I and III procollagen, fibronectin, and α-smooth muscle actin (α-SMA) mRNA, production of free radical and TGF-β1 protein, and positive staining of lumican in diaphragmatic fiber. Mechanical ventilation of lumican deficient mice attenuated diaphragmatic injury, type I and III procollagen, fibronectin, and α-SMA mRNA, and production of free radical and TGF-β1 protein. No significant diaphragmatic injury was found in mice subjected to normal tidal volume mechanical ventilation.
Conclusion: Our data showed that high tidal volume mechanical ventilation induced TGF-β1 production, TGF-β1-inducible genes, e.g., collagen, and diaphragmatic dysfunction through activation of the lumican.
Conflict of interest statement
Figures


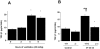



Similar articles
-
Lumican regulates ventilation-induced epithelial-mesenchymal transition through extracelluar signal-regulated kinase pathway.Chest. 2013 May;143(5):1252-1260. doi: 10.1378/chest.12-2058. Chest. 2013. PMID: 23154825 Free PMC article.
-
High tidal volume mechanical ventilation elicits increased activity in protein kinase B and c-Jun NH2-terminal kinase pathways in mouse diaphragm.Intensive Care Med. 2011 Dec;37(12):2015-22. doi: 10.1007/s00134-011-2350-x. Epub 2011 Sep 20. Intensive Care Med. 2011. PMID: 21932137
-
Lumican is increased in experimental and clinical heart failure, and its production by cardiac fibroblasts is induced by mechanical and proinflammatory stimuli.FEBS J. 2013 May;280(10):2382-98. doi: 10.1111/febs.12235. Epub 2013 Apr 2. FEBS J. 2013. PMID: 23480731
-
Epithelial repair: roles of extracellular matrix.Cornea. 2002 Mar;21(2 Suppl 1):S23-9. doi: 10.1097/00003226-200203001-00006. Cornea. 2002. PMID: 11995806 Review.
-
Functions of lumican and fibromodulin: lessons from knockout mice.Glycoconj J. 2002 May-Jun;19(4-5):287-93. doi: 10.1023/A:1025348417078. Glycoconj J. 2002. PMID: 12975607 Review.
Cited by
-
PEEP application during mechanical ventilation contributes to fibrosis in the diaphragm.Respir Res. 2023 Feb 13;24(1):46. doi: 10.1186/s12931-023-02356-y. Respir Res. 2023. PMID: 36782202 Free PMC article.
-
Suppression of Ventilation-Induced Diaphragm Fibrosis through the Phosphoinositide 3-Kinase-γ in a Murine Bleomycin-Induced Acute Lung Injury Model.Int J Mol Sci. 2024 Jun 8;25(12):6370. doi: 10.3390/ijms25126370. Int J Mol Sci. 2024. PMID: 38928077 Free PMC article.
-
Lumican regulates ventilation-induced epithelial-mesenchymal transition through extracelluar signal-regulated kinase pathway.Chest. 2013 May;143(5):1252-1260. doi: 10.1378/chest.12-2058. Chest. 2013. PMID: 23154825 Free PMC article.
-
Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome.Anesthesiology. 2014 Jul;121(1):189-98. doi: 10.1097/ALN.0000000000000264. Anesthesiology. 2014. PMID: 24732023 Free PMC article. Review.
References
-
- Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. - PubMed
-
- Chaudhary NI, Schnapp A, Park JE. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med. 2006;173:769–776. - PubMed
-
- Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167:1027–1035. - PubMed
-
- Zergeroglu MA, McKenzie MJ, Shanely RA, Van Gammeren D, DeRuisseau KC, et al. Mechanical ventilation-induced oxidative stress in the diaphragm. J Appl Physiol. 2003;95:1116–1124. - PubMed
-
- Li LF, Liao SK, Huang CC, Hung MJ, Quinn DA. Serine/threonine kinase-protein kinase B and extracellular signal-regulated kinase regulate ventilator-induced pulmonary fibrosis after bleomycin-induced acute lung injury: a prospective, controlled animal experiment. Crit Care. 2008;12:R103. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

