Increased virulence of an epidemic strain of Mycobacterium massiliense in mice
- PMID: 21931831
- PMCID: PMC3171484
- DOI: 10.1371/journal.pone.0024726
Increased virulence of an epidemic strain of Mycobacterium massiliense in mice
Abstract
Background: Chronic pulmonary disease and skin/soft tissue infections due to non-tuberculous mycobacteria (NTM) of the Mycobacterium chelonae-abscessus-massiliense group is an emerging health problem worldwide. Moreover, the cure rate for the infections this group causes is low despite aggressive treatment. Post-surgical outbreaks that reached epidemic proportions in Brazil recently were caused by M. massiliense isolates resistant to high-level disinfection with glutaraldehyde (GTA). Understanding the differences in the virulence and host immune responses induced by NTM differing in their sensitivity to disinfectants, and therefore their relative threat of causing outbreaks in hospitals, is an important issue.
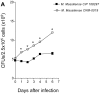
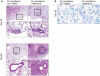
Methodology/principal finding: We compared the replication and survival inside macrophages of a GTA-susceptible reference Mycobacterium massiliense clinical isolate CIP 108297 and an epidemic strain from Brazil, CRM-0019, and characterized the immune responses of IFNγ knockout mice exposed to a high dose aerosol with these two isolates. CRM-0019 replicated more efficiently than CIP 108297 inside mouse bone marrow macrophages. Moreover, the animals infected with CRM-0019 showed a progressive lung infection characterized by a delayed influx of CD4+ and CD8+ T cells, culminating in extensive lung consolidation and demonstrated increased numbers of pulmonary CD4+ Foxp3+ regulatory T cells compared to those infected with the reference strain. Immunosuppressive activity of regulatory T cells may contribute to the progression and worsening of NTM disease by preventing the induction of specific protective immune responses.
Conclusions/significance: These results provide the first direct evidence of the increased virulence in macrophages and mice and pathogenicity in vivo of the Brazilian epidemic isolate and the first observation that NTM infections can be associated with variable levels of regulatory T cells which may impact on their virulence and ability to persist in the host.
Conflict of interest statement
Figures
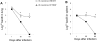
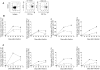
Similar articles
-
Extrapulmonary infections caused by a dominant strain of Mycobacterium massiliense (Mycobacterium abscessus subspecies bolletii).Clin Microbiol Infect. 2013 Oct;19(10):E473-82. doi: 10.1111/1469-0691.12261. Epub 2013 May 30. Clin Microbiol Infect. 2013. PMID: 23718188
-
High virulent clinical isolates of Mycobacterium abscessus from patients with the upper lobe fibrocavitary form of pulmonary disease.Microb Pathog. 2009 Dec;47(6):321-8. doi: 10.1016/j.micpath.2009.09.010. Epub 2009 Oct 2. Microb Pathog. 2009. PMID: 19800962
-
Acute immune response to Mycobacterium massiliense in C57BL/6 and BALB/c mice.Infect Immun. 2010 Apr;78(4):1571-81. doi: 10.1128/IAI.00731-09. Epub 2010 Feb 1. Infect Immun. 2010. PMID: 20123718 Free PMC article.
-
Host immune response to rapidly growing mycobacteria, an emerging cause of chronic lung disease.Am J Respir Cell Mol Biol. 2010 Oct;43(4):387-93. doi: 10.1165/rcmb.2009-0276TR. Epub 2010 Jan 15. Am J Respir Cell Mol Biol. 2010. PMID: 20081053 Review.
-
T cell mediated immunity to Mycobacterium tuberculosis.Curr Opin Microbiol. 1999 Feb;2(1):89-93. doi: 10.1016/s1369-5274(99)80015-0. Curr Opin Microbiol. 1999. PMID: 10047556 Review.
Cited by
-
Increased survival and proliferation of the epidemic strain Mycobacterium abscessus subsp. massiliense CRM0019 in alveolar epithelial cells.BMC Microbiol. 2017 Sep 13;17(1):195. doi: 10.1186/s12866-017-1102-7. BMC Microbiol. 2017. PMID: 28903728 Free PMC article.
-
Antimycobacterial Activity of a New Peptide Polydim-I Isolated from Neotropical Social Wasp Polybia dimorpha.PLoS One. 2016 Mar 1;11(3):e0149729. doi: 10.1371/journal.pone.0149729. eCollection 2016. PLoS One. 2016. PMID: 26930596 Free PMC article.
-
Different immunosuppressive mechanisms in multi-drug-resistant tuberculosis and non-tuberculous mycobacteria patients.Clin Exp Immunol. 2013 Feb;171(2):210-9. doi: 10.1111/cei.12007. Clin Exp Immunol. 2013. PMID: 23286948 Free PMC article.
-
Comparing the Utilities of Different Multilocus Sequence Typing Schemes for Identifying Outbreak Strains of Mycobacterium abscessus subsp. massiliense.J Clin Microbiol. 2019 Dec 23;58(1):e01304-19. doi: 10.1128/JCM.01304-19. Print 2019 Dec 23. J Clin Microbiol. 2019. PMID: 31619535 Free PMC article.
-
Genomic epidemiology of a national outbreak of post-surgical Mycobacterium abscessus wound infections in Brazil.Microb Genom. 2017 May 3;3(5):e000111. doi: 10.1099/mgen.0.000111. eCollection 2017 May. Microb Genom. 2017. PMID: 28884021 Free PMC article.
References
-
- Adekambi T, Berger P, Raoult D, Drancourt M. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii, Mycobacterium phocaicum and Mycobacterium aubagnense. Int J Syst Evol Microbiol. 2006;56:133–143. - PubMed
-
- Wallace RJ, Jr, Brown BA, Griffith DE. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol. 1998;52:453–490. - PubMed
-
- Phillips MS, Fordham von Reyn C. Nosocomial infections due to nontuberculous mycobacteria. Clinical Infectious Diseases. 2001;33:1363–1374. - PubMed
-
- Holland SM. Nontuberculous mycobacteria. Am J Med Sci. 2001;321:49–55. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

