NEK1 Facilitates Cohesin Removal during Mammalian Spermatogenesis
- PMID: 21931878
- PMCID: PMC3175124
- DOI: 10.3390/genes2010260
NEK1 Facilitates Cohesin Removal during Mammalian Spermatogenesis
Abstract
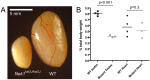
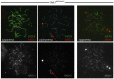
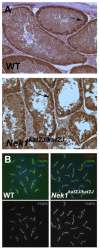
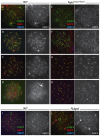
Meiosis is a highly conserved process, which is stringently regulated in all organisms, from fungi through to humans. Two major events define meiosis in eukaryotes. The first is the pairing, or synapsis, of homologous chromosomes and the second is the exchange of genetic information in a process called meiotic recombination. Synapsis is mediated by the meiosis-specific synaptonemal complex structure in combination with the cohesins that tether sister chromatids together along chromosome arms through prophase I. Previously, we identified FKBP6 as a novel component of the mammalian synaptonemal complex. Further studies demonstrated an interaction between FKBP6 and the NIMA-related kinase-1, NEK1. To further investigate the role of NEK1 in mammalian meiosis, we have examined gametogenesis in the spontaneous mutant, Nek1kat2J. Homozygous mutant animals show decreased testis size, defects in testis morphology, and in cohesin removal at late prophase I of meiosis, causing complete male infertility. Cohesin protein SMC3 remains localized to the meiotic chromosome cores at diplonema in the Nek1 mutant, and also in the related Fkbp6 mutant, while in wild type cells SMC3 is removed from the cores at the end of prophase I and becomes more diffuse throughout the DAPI stained region of the nucleus. These data implicate NEK1 as a possible kinase involved in cohesin redistribution in murine spermatocytes.
Figures




References
-
- Handel M.A., Schimenti J.C. Genetics of mammalian meiosis: Regulation, dynamics and impact on fertility. Nat. Rev. Genet. 2010;11:124–136. - PubMed
-
- Hassold T., Hunt P. To err (meiotically) is human: The genesis of human aneuploidy. Nat. Rev. Genet. 2001;2:280–291. - PubMed
-
- Hassold T., Hunt P.A., Sherman S. Trisomy in humans: Incidence, origin and etiology. Curr. Opin. Genet. Dev. 1993;3:398–403. - PubMed
-
- Hassold T., Abruzzo M., Adkins K., Griffin D., Merrill M., Millie E., Saker D., Shen J., Zaragoza M. Human aneuploidy: Incidence, origin, and etiology. Environ. Mol. Mutagen. 1996;28:167–175. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
