Calcification of primary human osteoblast cultures under flow conditions using polycaprolactone scaffolds for intravascular applications
- PMID: 21932279
- PMCID: PMC3244545
- DOI: 10.1002/term.472
Calcification of primary human osteoblast cultures under flow conditions using polycaprolactone scaffolds for intravascular applications
Abstract
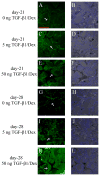
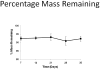
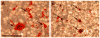
Total atherosclerotic occlusion is a leading cause of death. Recent animal models of this disease are devoid of cell-mediated calcification and arteries are often not occluded gradually. This study is part of a project with the objective of developing a new model featuring the above two characteristics, using a tissue-engineering scaffold. The amount and distribution of calcium deposits in primary human osteoblast (HOB) cultures on polycaprolactone (PCL) scaffolds under flow conditions were investigated. HOBs were cultured on PCL scaffolds with TGF-β1 loadings of 0 (control), 5 and 50 ng. HOB-PCL constructs were cultured in spinner flasks. Under flow conditions, cell numbers present in HOB cultures on PCL scaffolds increased from day 7 to day 14, and most calcification was induced at day 21. TGF-β1 loadings of 5 and 50 ng did not show a significant difference in ALP activity, cell numbers and amount of calcium deposited in HOB cultures, but calcium staining showed that 50 ng TGF-β1 had higher calcium deposited on both days 21 and 28 under flow conditions compared with 5 ng of loading. Amount of calcium deposited by HOBs on day 28 showed a decrease from their levels on day 21. PCL degradation may be a factor contributing to this loss. The results indicate that cell-induced calcification can be achieved on PCL scaffolds under flow conditions. In conclusion, TGFβ1-HOB loaded PCL can be applied to create a model for total atherosclerotic occlusion with cell-deposited calcium in animal arteries.
Keywords: PCL; calcification; dynamic flow; polycaprolactone; primary human osteoblast; scaffold; tissue engineering; total atherosclerotic occlusion.
Copyright © 2011 John Wiley & Sons, Ltd.
Figures
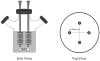
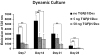
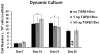
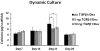
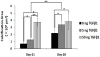
References
-
- Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24(7):1161–1170. - PubMed
-
- Agrawal CM, McKinney JS, Lanctot D, Athanasiou KA. Effects of fluid flow on the in vitro degradation kinetics of biodegradable scaffolds for tissue engineering. Biomaterials. 2000;21(23):2443–2452. - PubMed
-
- Alexopoulos N, Raggi P. Calcification in atherosclerosis. Nat Rev Cardiol. 2009;6 (11):681–688. - PubMed
-
- Barralet JE, Wallace LL, Strain AJ. Tissue engineering of human biliary epithelial cells on polyglycolic acid/polycaprolactone scaffolds maintains long-term phenotypic stability. Tissue Eng. 2003;9(5):1037–1045. - PubMed
-
- Bilgen B, Barabino GA. Location of scaffolds in bioreactors modulates the hydrodynamic environment experienced by engineered tissues. Biotechnol Bioeng. 2007;98(1):282–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

