Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes
- PMID: 21932398
- PMCID: PMC3245352
- DOI: 10.1002/hep.24681
Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes
Abstract
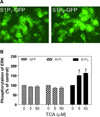
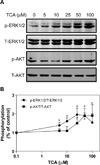
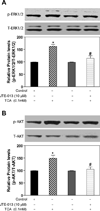
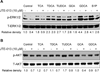
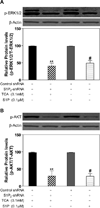
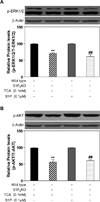
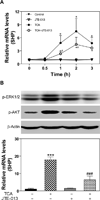
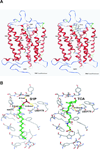
Bile acids have been shown to be important regulatory molecules for cells in the liver and gastrointestinal tract. They can activate various cell signaling pathways including extracellular regulated kinase (ERK)1/2 and protein kinase B (AKT) as well as the G-protein-coupled receptor (GPCR) membrane-type bile acid receptor (TGR5/M-BAR). Activation of the ERK1/2 and AKT signaling pathways by conjugated bile acids has been reported to be sensitive to pertussis toxin (PTX) and dominant-negative Gα(i) in primary rodent hepatocytes. However, the GPCRs responsible for activation of these pathways have not been identified. Screening GPCRs in the lipid-activated phylogenetic family (expressed in HEK293 cells) identified sphingosine-1-phosphate receptor 2 (S1P(2) ) as being activated by taurocholate (TCA). TCA, taurodeoxycholic acid (TDCA), tauroursodeoxycholic acid (TUDCA), glycocholic acid (GCA), glycodeoxycholic acid (GDCA), and S1P-induced activation of ERK1/2 and AKT were significantly inhibited by JTE-013, a S1P(2) antagonist, in primary rat hepatocytes. JTE-013 significantly inhibited hepatic ERK1/2 and AKT activation as well as short heterodimeric partner (SHP) mRNA induction by TCA in the chronic bile fistula rat. Knockdown of the expression of S1P(2) by a recombinant lentivirus encoding S1P(2) shRNA markedly inhibited the activation of ERK1/2 and AKT by TCA and S1P in rat primary hepatocytes. Primary hepatocytes prepared from S1P(2) knock out (S1P(2) (-/-) ) mice were significantly blunted in the activation of the ERK1/2 and AKT pathways by TCA. Structural modeling of the S1P receptors indicated that only S1P(2) can accommodate TCA binding. In summary, all these data support the hypothesis that conjugated bile acids activate the ERK1/2 and AKT signaling pathways primarily through S1P(2) in primary rodent hepatocytes.
Copyright © 2011 American Association for the Study of Liver Diseases.
Figures
References
-
- Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. - PubMed
-
- Nguyen A, Bouscarel B. Bile acids and signal transduction: role in glucose homeostasis. Cell Signal. 2008;20:2180–2197. - PubMed
-
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. - PubMed
-
- Zhang Y, Edwards PA. FXR signaling in metabolic disease. FEBS Lett. 2008;582:10–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
