CD59 incorporation protects hepatitis C virus against complement-mediated destruction
- PMID: 21932413
- PMCID: PMC3417136
- DOI: 10.1002/hep.24686
CD59 incorporation protects hepatitis C virus against complement-mediated destruction
Abstract
Several enveloped viruses including human immunodeficiency virus type 1 (HIV-1), cytomegalovirus (CMV), herpes simplex virus 1 (HSV-1), Ebola virus, vaccinia virus, and influenza virus have been found to incorporate host regulators of complement activation (RCA) into their viral envelopes and, as a result, escape antibody-dependent complement-mediated lysis (ADCML). Hepatitis C virus (HCV) is an enveloped virus of the family Flaviviridae and incorporates more than 10 host lipoproteins. Patients chronically infected with HCV develop high-titer and crossreactive neutralizing antibodies (nAbs), yet fail to clear the virus, raising the possibility that HCV may also use the similar strategy of RCA incorporation to escape ADCML. The current study was therefore undertaken to determine whether HCV virions incorporate biologically functional CD59, a key member of RCA. Our experiments provided several lines of evidence demonstrating that CD59 was associated with the external membrane of HCV particles derived from either Huh7.5.1 cells or plasma samples from HCV-infected patients. First, HCV particles were captured by CD59-specific Abs. Second, CD59 was detected in purified HCV particles by immunoblot analysis and in the cell-free supernatant from HCV-infected Huh7.5.1 cells, but not from uninfected or adenovirus serotype 5 (Ad5) (a nonenveloped cytolytic virus)-infected Huh7.5.1 cells by enzyme-linked immunosorbent assay. Last, abrogation of CD59 function with its blockers increased the sensitivity of HCV virions to ADCML, resulting in a significant reduction of HCV infectivity. Additionally, direct addition of CD59 blockers into plasma samples from HCV-infected patients increased autologous virolysis.
Conclusion: Our study, for the first time, demonstrates that CD59 is incorporated into both cell line-derived and plasma primary HCV virions at levels that protect against ADCML. This is also the first report to show that direct addition of RCA blockers into plasma from HCV-infected patients renders endogenous plasma virions sensitive to ADCML.
Copyright © 2011 American Association for the Study of Liver Diseases.
Figures

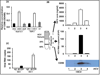




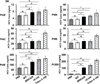

References
-
- Spear GT, et al. Host cell-derived complement control proteins CD55 and CD59 are incorporated into the virions of two unrelated enveloped viruses. Human T cell leukemia/lymphoma virus type I (HTLV-I) and human cytomegalovirus (HCMV) J Immunol. 1995;155:4376–4381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
