Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers
- PMID: 21932809
- PMCID: PMC3219056
- DOI: 10.1021/ar2001777
Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers
Abstract
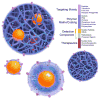
For decades, researchers and medical professionals have aspired to develop mechanisms for noninvasive treatment and monitoring of pathological conditions within the human body. The emergence of nanotechnology has spawned new opportunities for novel drug delivery vehicles capable of concomitant detection, monitoring, and localized treatment of specific disease sites. In turn, researchers have endeavored to develop an imaging moiety that could be functionalized to seek out specific diseased conditions and could be monitored with conventional clinical imaging modalities. Such nanoscale detection systems have the potential to increase early detection of pathophysiological conditions because they can detect abnormal cells before they even develop into diseased tissue or tumors. Ideally, once the diseased cells are detected, clinicians would like to treat those cells simultaneously. This idea led to the concept of multifunctional carriers that could target, detect, and treat diseased cells. The term "theranostics" has been created to describe this promising area of research that focuses on the combination of diagnostic detection agents with therapeutic drug delivery carriers. Targeted theranostic nanocarriers offer an attractive improvement to disease treatment because of their ability to execute simultaneous functions at targeted diseased sites. Research efforts in the field of theranostics encompass a broad variety of drug delivery vehicles, imaging contrast agents, and targeting modalities for the development of an all-in-one, localized detection and treatment system. Nanotheranostic systems that utilize metallic or magnetic imaging nanoparticles can also be used as thermal therapeutic systems. This Account explores recent advances in the field of nanotheranostics and the various fundamental components of an effective theranostic carrier.
Figures


Similar articles
-
Mechanized silica nanoparticles: a new frontier in theranostic nanomedicine.Acc Chem Res. 2011 Oct 18;44(10):903-13. doi: 10.1021/ar200018x. Epub 2011 Jun 15. Acc Chem Res. 2011. PMID: 21675720 Free PMC article. Review.
-
Voyage of theranostic liposomes for imaging and therapy.J Cosmet Laser Ther. 2017 Aug;19(4):245-249. doi: 10.1080/14764172.2017.1279331. Epub 2017 Jan 31. J Cosmet Laser Ther. 2017. PMID: 28135901 Review.
-
Theranostic magnetic nanoparticles.Acc Chem Res. 2011 Oct 18;44(10):863-74. doi: 10.1021/ar200085c. Epub 2011 Aug 8. Acc Chem Res. 2011. PMID: 21823593 Review.
-
Theranostic nanogels: multifunctional agents for simultaneous therapeutic delivery and diagnostic imaging.Nanoscale. 2024 Jul 25;16(29):14033-14056. doi: 10.1039/d4nr01423e. Nanoscale. 2024. PMID: 38990143 Review.
-
Theranostic nanoparticles engineered for clinic and pharmaceutics.Acc Chem Res. 2011 Oct 18;44(10):1114-22. doi: 10.1021/ar2000056. Epub 2011 Jul 6. Acc Chem Res. 2011. PMID: 21732606 Review.
Cited by
-
Nanobottles for Controlled Release and Drug Delivery.Adv Healthc Mater. 2021 Feb;10(4):e2000587. doi: 10.1002/adhm.202000587. Epub 2020 Jun 16. Adv Healthc Mater. 2021. PMID: 32543127 Free PMC article. Review.
-
Controlled Release of Molecules to Enhance Cell Survival and Regeneration.Methods Mol Biol. 2025;2848:259-267. doi: 10.1007/978-1-0716-4087-6_16. Methods Mol Biol. 2025. PMID: 39240528
-
pH-Sensitive O6-Benzylguanosine Polymer Modified Magnetic Nanoparticles for Treatment of Glioblastomas.Bioconjug Chem. 2017 Jan 18;28(1):194-202. doi: 10.1021/acs.bioconjchem.6b00545. Epub 2016 Dec 12. Bioconjug Chem. 2017. PMID: 27936607 Free PMC article.
-
Stimuli-Responsive Polymeric Nanoplatforms for Cancer Therapy.Front Bioeng Biotechnol. 2021 Jun 25;9:707319. doi: 10.3389/fbioe.2021.707319. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34249894 Free PMC article. Review.
-
Surface hydrolysis-mediated PEGylation of poly(N-isopropyl acrylamide) based nanogels.Regen Biomater. 2017 Oct;4(5):281-287. doi: 10.1093/rb/rbx022. Epub 2017 Aug 7. Regen Biomater. 2017. PMID: 29026641 Free PMC article.
References
-
- Islam T, Josephson L. Current state and future applications of active targeting in malignancies using superparamagnetic iron oxide nanoparticles. Cancer Biomarkers. 2009;5:99–107. - PubMed
-
- Thorek DLJ, Chen A, Czupryna J, Tsourkas A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Annals of Biomedical Engineering. 2006;34:23–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

