Substitution of the human αC region with the analogous chicken domain generates a fibrinogen with severely impaired lateral aggregation: fibrin monomers assemble into protofibrils but protofibrils do not assemble into fibers
- PMID: 21932842
- PMCID: PMC3203410
- DOI: 10.1021/bi201094v
Substitution of the human αC region with the analogous chicken domain generates a fibrinogen with severely impaired lateral aggregation: fibrin monomers assemble into protofibrils but protofibrils do not assemble into fibers
Abstract
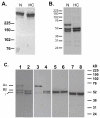
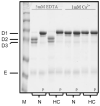
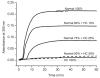
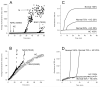
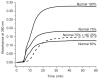
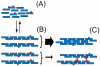
Fibrin polymerization occurs in two steps: the assembly of fibrin monomers into protofibrils and the lateral aggregation of protofibrils into fibers. Here we describe a novel fibrinogen that apparently impairs only lateral aggregation. This variant is a hybrid, where the human αC region has been replaced with the homologous chicken region. Several experiments indicate this hybrid human-chicken (HC) fibrinogen has an overall structure similar to normal. Thrombin-catalyzed fibrinopeptide release from HC fibrinogen was normal. Plasmin digests of HC fibrinogen produced fragments that were similar to normal D and E; further, as with normal fibrinogen, the knob 'A' peptide, GPRP, reversed the plasmin cleavage associated with addition of EDTA. Dynamic light scattering and turbidity studies with HC fibrinogen showed polymerization was not normal. Whereas early small increases in hydrodynamic radius and absorbance paralleled the increases seen during the assembly of normal protofibrils, HC fibrinogen showed no dramatic increase in scattering as observed with normal lateral aggregation. To determine whether HC and normal fibrinogen could form a copolymer, we examined mixtures of these. Polymerization of normal fibrinogen was markedly changed by HC fibrinogen, as expected for mixed polymers. When the mixture contained 0.45 μM normal and 0.15 μM HC fibrinogen, the initiation of lateral aggregation was delayed and the final fiber size was reduced relative to normal fibrinogen at 0.45 μM. Considered altogether, our data suggest that HC fibrin monomers can assemble into protofibrils or protofibril-like structures, but these either cannot assemble into fibers or assemble into very thin fibers.
Figures
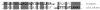

References
-
- Kollman JM, Pandi L, Sawaya MR, Riley M, Doolittle RF. Crystal Structure of Human Fibrinogen. Biochemistry. 2009;48:3877–3886. - PubMed
-
- Weisel JW, Medved L. The structure and function of the alpha C domains of fibrinogen. Ann N Y Acad Sci. 2001;936:312–327. - PubMed
-
- Medved LV, Gorkun OV, Privalov PL. Structural organization of C-terminal parts of fibrinogen A alpha-chains. FEBS Letters. 1983;160:291–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

