Compartmental hollow fiber capillary membrane-based bioreactor technology for in vitro studies on red blood cell lineage direction of hematopoietic stem cells
- PMID: 21933020
- PMCID: PMC3262978
- DOI: 10.1089/ten.TEC.2011.0305
Compartmental hollow fiber capillary membrane-based bioreactor technology for in vitro studies on red blood cell lineage direction of hematopoietic stem cells
Abstract
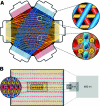
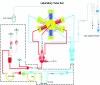
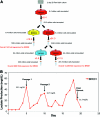
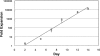
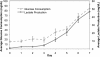
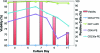
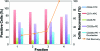
Continuous production of red blood cells (RBCs) in an automated closed culture system using hematopoietic stem cell (HSC) progenitor cell populations is of interest for clinical application because of the high demand for blood transfusions. Previously, we introduced a four-compartment bioreactor that consisted of two bundles of hollow fiber microfiltration membranes for transport of culture medium (forming two medium compartments), interwoven with one bundle of hollow fiber membranes for transport of oxygen (O(2)), carbon dioxide (CO(2)), and other gases (forming one gas compartment). Small-scale prototypes were developed of the three-dimensional (3D) perfusion cell culture systems, which enable convection-based mass transfer and integral oxygenation in the cell compartment. CD34(+) HSC were isolated from human cord blood units using a magnetic separation procedure. Cells were inoculated into 2- or 8-mL scaled-down versions of the previously designed 800-mL cell compartment devices and perfused with erythrocyte proliferation and differentiation medium. First, using the small-scale 2-mL analytical scale bioreactor, with an initial seeding density of 800,000 cells/mL, we demonstrated approximately 100-fold cell expansion and differentiation after 7 days of culture. An 8-mL laboratory-scale bioreactor was then used to show pseudocontinuous production by intermediately harvesting cells. Subsequently, we were able to use a model to demonstrate semicontinuous production with up to 14,288-fold expansion using seeding densities of 800,000 cells/mL. The down-scaled culture technology allows for expansion of CD34(+) cells and stimulating these progenitors towards RBC lineage, expressing approximately 40% CD235(+) and enucleation. The 3D perfusion technology provides an innovative tool for studies on RBC production, which is scalable.
Figures




References
-
- Bersch C. The give and take of blood banking. Medical Laboratory Observer [on-line] Mar, 2010. http://www.mlo-online.com/ http://www.mlo-online.com/ - PubMed
-
- Yap C. Lau L. Krishnaswamy M. Gaskell M. Yii M. Age of transfused red cells and early outcomes after cardiac surgery. Ann Thorac Surg. 2008;86:554. - PubMed
-
- Kirouac D.C. Zandstra P.W. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3:369. - PubMed
-
- Noll T. Jelonek N. Schmidt S. Biselli M. Wandrey C. Cultivation of hematopoietic stem and progenitor cells: biochemical engineering aspects. Adv Biochem Eng Biotechnol. 2002;74:111. - PubMed
-
- Kowalczyk M. Waldron K. Kresnowati P. Danquah M.K. Review: process challenges relating to hematopoietic stem cell culture in bioreactors. J. Ind Microbiol Biotechnol. 2011;38:761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

