Control dominating subclones for managing cancer progression and posttreatment recurrence by subclonal switchboard signal: implication for new therapies
- PMID: 21933025
- PMCID: PMC6916525
- DOI: 10.1089/scd.2011.0267
Control dominating subclones for managing cancer progression and posttreatment recurrence by subclonal switchboard signal: implication for new therapies
Abstract
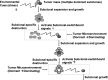
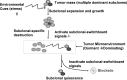
In contrast to hematological malignancies, meaningful improvements in survival statistics for patients with malignant brain tumors have not been realized in >40 years of clinical research. Clearly, a new medical approach to brain cancers is needed. Recent research has led to a new concept that needs to destroy all cancer subclones to control the cancer progression. However, this new concept fails to distinguish the difference between dominating subclones and dormant subclones. Here, we address the issue of clonal switch and emphasize that there may be one or more than one dominant clones within the tumor mass at any time. Destructing one dominant clone triggers activating other dormant subclones to become dominating subclones, causing cancer progress and post-treatment cancer recurrence. We postulate the concept of subclonal switchboard signaling and the pathway that involved in this process. In the context of stem cell and development, there is a parallel with the concept of quiescent/dormant cancer stem cells (CSC) and their progeny, the differentiated cancer cells; these 2 populations communicate and co-exist. The mechanism with which determines to extend self-renewal and expansion of CSC is needed to elucidate. We suggest eliminating the "dominating subclonal switchboard signals" that shift the dormant subclones to dominating subclones as a new strategy.
Figures
References
-
- Drake N. Forty years on from Nixon's war, cancer research “evolves”. Nat Med. 2011;17:757. - PubMed
-
- Burgess DJ. Cancer genetics: initially complex, always heterogeneous. Nat Rev Genet. 2011;12:154–155. - PubMed
-
- Anderson K. Lutz C. van Delft FW. Bateman CM. Guo Y. Colman SM. Kempski H. Moorman AV. Titley I, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469:356–361. - PubMed
-
- Notta F. Mullighan CG. Wang JC. Poeppl A. Doulatov S. Phillips LA. Ma J. Minden MD. Downing JR. Dick JE. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature. 2011;469:362–367. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

