Regioselective sulfation and glucuronidation of phenolics: insights into the structural basis
- PMID: 21933112
- PMCID: PMC3426368
- DOI: 10.2174/138920011797470100
Regioselective sulfation and glucuronidation of phenolics: insights into the structural basis
Abstract
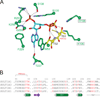
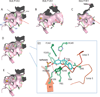
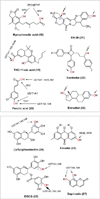
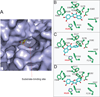
The phase II metabolism sulfation and glucuronidation, mediated by sulfotransferases (SULTs) and UDP-glucuronosyltransferases (UGTs) respectively, are significant metabolic pathways for numerous endo-and xenobiotics. Understanding of SULT/UGT substrate specificity including regioselectivity (i.e., position preference) is of great importance in predicting contribution of sulfation/ glucuronidation to drug and metabolite disposition in vivo. This review summarizes regioselective sulfation and glucuronidation of phenolic compounds with multiple hydroxyl (OH) groups as the potential conjugation sites. The strict regioselective patterns are highlighted for several SULT and UGT isoforms towards flavonoids, a large class of natural polyphenols. To seek for a molecular-level explanation, the enzyme structures (i.e., SULT crystal structures and a homology-modeled UGT structure) combined with molecular docking are employed. In particular, the structural basis for regioselective metabolism of flavonoids by SULT1A3 and UGT1A1 is discussed. It is concluded that the regioselective nature of these phase II enzymes is determined by the size and shape of the binding pocket. While the molecular structures of the enzymes can be used to explain regioselective metabolism regarding the binding property, predicting the turnover at different positions remains a particularly difficult task.
Figures




Similar articles
-
Uridine diphosphate glucuronosyltransferase isoform-dependent regiospecificity of glucuronidation of flavonoids.J Agric Food Chem. 2011 Jul 13;59(13):7452-64. doi: 10.1021/jf1041454. Epub 2011 Jun 6. J Agric Food Chem. 2011. PMID: 21413806 Free PMC article.
-
Simultaneous determination of sulfation and glucuronidation of flavones in FVB mouse intestine in vitro and in vivo.J Appl Toxicol. 2013 Apr;33(4):273-80. doi: 10.1002/jat.1737. Epub 2011 Dec 15. J Appl Toxicol. 2013. PMID: 22174032
-
Application of ultra high-performance liquid chromatography tandem mass spectrometry to investigate the regioselective glucuronidation of icaritin in vitro.J Pharm Biomed Anal. 2018 May 30;154:444-453. doi: 10.1016/j.jpba.2018.02.029. Epub 2018 Feb 13. J Pharm Biomed Anal. 2018. PMID: 29587224
-
Sulfation and glucuronidation of phenols: implications in coenyzme Q metabolism.Methods Enzymol. 2005;400:342-59. doi: 10.1016/S0076-6879(05)00020-0. Methods Enzymol. 2005. PMID: 16399359 Review.
-
First-pass metabolism via UDP-glucuronosyltransferase: a barrier to oral bioavailability of phenolics.J Pharm Sci. 2011 Sep;100(9):3655-81. doi: 10.1002/jps.22568. Epub 2011 Apr 11. J Pharm Sci. 2011. PMID: 21484808 Free PMC article. Review.
Cited by
-
Discovery of Potent, Selective Triazolothiadiazole-Containing c-Met Inhibitors.ACS Med Chem Lett. 2021 May 24;12(6):955-960. doi: 10.1021/acsmedchemlett.1c00094. eCollection 2021 Jun 10. ACS Med Chem Lett. 2021. PMID: 34141080 Free PMC article.
-
Phenolic acid phenethylesters and their corresponding ketones: Inhibition of 5-lipoxygenase and stability in human blood and HepaRG cells.Pharmacol Res Perspect. 2019 Sep 13;7(5):e00524. doi: 10.1002/prp2.524. eCollection 2019 Oct. Pharmacol Res Perspect. 2019. PMID: 31523435 Free PMC article.
-
Dietary Polyphenols as a Protection against Cognitive Decline: Evidence from Animal Experiments; Mechanisms and Limitations.Antioxidants (Basel). 2023 May 5;12(5):1054. doi: 10.3390/antiox12051054. Antioxidants (Basel). 2023. PMID: 37237920 Free PMC article. Review.
-
In Vitro Glucuronidation and Sulfation of ε-Viniferin, a Resveratrol Dimer, in Humans and Rats.Molecules. 2017 May 3;22(5):733. doi: 10.3390/molecules22050733. Molecules. 2017. PMID: 28467376 Free PMC article.
-
Properties of Dietary Flavone Glycosides, Aglycones, and Metabolites on the Catalysis of Human Endoplasmic Reticulum Uridine Diphosphate Glucuronosyltransferase 2B7 (UGT2B7).Nutrients. 2023 Nov 28;15(23):4941. doi: 10.3390/nu15234941. Nutrients. 2023. PMID: 38068799 Free PMC article.
References
-
- Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26(8):1001–1043. - PubMed
-
- Ibrahim KE, Midgley JM, Crowley JR, Williams CM. The Mammalian Metabolism of R-(-)-m-Synephrine. J Pharm Pharmacol. 1983;35(3):144–147. - PubMed
-
- Gumbhir K. An Investigation of Pharmacokinetics of Phenylephrine and its Metabolites in Humans. University of Missouri-Kansas City: Pharmaceutical Sciences; 1993. p. 216.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

