Regioselective sulfation and glucuronidation of phenolics: insights into the structural basis
- PMID: 21933112
- PMCID: PMC3426368
- DOI: 10.2174/138920011797470100
Regioselective sulfation and glucuronidation of phenolics: insights into the structural basis
Abstract
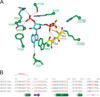
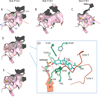
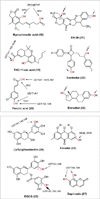
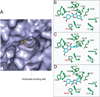
The phase II metabolism sulfation and glucuronidation, mediated by sulfotransferases (SULTs) and UDP-glucuronosyltransferases (UGTs) respectively, are significant metabolic pathways for numerous endo-and xenobiotics. Understanding of SULT/UGT substrate specificity including regioselectivity (i.e., position preference) is of great importance in predicting contribution of sulfation/ glucuronidation to drug and metabolite disposition in vivo. This review summarizes regioselective sulfation and glucuronidation of phenolic compounds with multiple hydroxyl (OH) groups as the potential conjugation sites. The strict regioselective patterns are highlighted for several SULT and UGT isoforms towards flavonoids, a large class of natural polyphenols. To seek for a molecular-level explanation, the enzyme structures (i.e., SULT crystal structures and a homology-modeled UGT structure) combined with molecular docking are employed. In particular, the structural basis for regioselective metabolism of flavonoids by SULT1A3 and UGT1A1 is discussed. It is concluded that the regioselective nature of these phase II enzymes is determined by the size and shape of the binding pocket. While the molecular structures of the enzymes can be used to explain regioselective metabolism regarding the binding property, predicting the turnover at different positions remains a particularly difficult task.
Figures




References
-
- Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26(8):1001–1043. - PubMed
-
- Ibrahim KE, Midgley JM, Crowley JR, Williams CM. The Mammalian Metabolism of R-(-)-m-Synephrine. J Pharm Pharmacol. 1983;35(3):144–147. - PubMed
-
- Gumbhir K. An Investigation of Pharmacokinetics of Phenylephrine and its Metabolites in Humans. University of Missouri-Kansas City: Pharmaceutical Sciences; 1993. p. 216.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

