Collagen type II and a thermo-responsive polymer of N-isopropylacrylamide induce arthritis independent of Toll-like receptors: a strong influence by major histocompatibility complex class II and Ncf1 genes
- PMID: 21933654
- PMCID: PMC3204090
- DOI: 10.1016/j.ajpath.2011.07.034
Collagen type II and a thermo-responsive polymer of N-isopropylacrylamide induce arthritis independent of Toll-like receptors: a strong influence by major histocompatibility complex class II and Ncf1 genes
Abstract
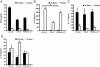
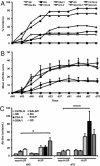
We established and characterized an arthritis mouse model using collagen type II (CII) and a thermo-responsive polymer, poly(N-isopropylacrylamide) (PNiPAAm). The new PNiPAAm adjuvant is TLR-independent, as all immunized TLR including MyD88-deficient mice developed an anti-CII response. Unlike other adjuvants, PNiPPAm did not skew the cytokine response (IL-1β, IFN-γ, IL-4, and IL-17), as there was no immune deviation towards any one type of immune spectrum after immunization with CII/PNiPPAm. Hence, using PNiPAAm, we studied the actual immune response to the self-protein, CII. We observed arthritis and autoimmunity development in several murine strains having different major histocompatibility complex (MHC) haplotypes after CII/PNiPAAm immunization but with a clear MHC association pattern. Interestingly, C57Bl/6 mice did not develop CII-induced arthritis, with PNiPAAm demonstrating absolute requirement for a classical adjuvant. Presence of a gene (Ncf1) mutation in the NADPH oxidation complex has a profound influence in arthritis and using PNiPAAm we could show that the high CIA severity in Ncf1 mutated mice is independent of any classical adjuvant. Macrophages, neutrophils, eosinophils, and osteoclasts but not mast cells dominated the inflamed joints. Furthermore, arthritis induction in the adjuvant-free, eosinophil-dependent Vβ12 DBA/1 mice could be shown to develop arthritis independent of eosinophils using CII/PNiPAAm. Thus, biocompatible and biodegradable PNiPAAm offers unique opportunities to study actual autoimmunity independent of TLR and a particular cytokine phenotype profile.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Brunsberg U., Gustafsson K., Jansson L., Michaëlsson E., &Ährlund-Richter L., Pettersson S., Mattsson R., Holmdahl R. Expression of a transgenic class II Ab gene confers susceptibility to collagen-induced arthritis. Eur J Immunol. 1994;24:1698–1702. - PubMed
-
- Wooley P.H., Luthra H.S., Griffiths M.M., Stuart J.M., Huse A., David C.S. Type II collagen induced arthritis in mice: IV. Variations in immunogenetic regulation provide evidence for multiple arthritogenic epitopes on the collagen molecule. J Immunol. 1985;135:2443–2451. - PubMed
-
- Campbell I.K., Hamilton J.A., Wicks I.P. Collagen-induced arthritis in C57BL/6 (H-2b) mice: new insights into an important disease model of rheumatoid arthritis. Eur J Immunol. 2000;30:1568–1575. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

