Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome
- PMID: 21933807
- PMCID: PMC3212714
- DOI: 10.1093/brain/awr234
Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome
Abstract
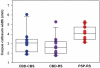
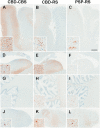
Patients with corticobasal degeneration can present with several different clinical syndromes, making ante-mortem diagnosis a challenge. Corticobasal syndrome is the clinical phenotype originally described for corticobasal degeneration, characterized by asymmetric rigidity and apraxia, cortical sensory deficits, dystonia and myoclonus. Some patients do not develop these features, but instead have clinical features consistent with the Richardson syndrome presentation of progressive supranuclear palsy, characterized by postural instability, early unexplained falls, vertical supranuclear gaze palsy, symmetric motor disability and dysphagia. The aim of this study was to identify differences in corticobasal degeneration presenting with corticobasal syndrome (n = 11) or Richardson syndrome (n = 15) with respect to demographic, clinical and neuropathological features. Corticobasal degeneration cases were also compared with patients with pathologically proven progressive supranuclear palsy with Richardson syndrome (n = 15). Cases with corticobasal degeneration, regardless of presentation, shared histopathological and tau biochemical characteristics, but they had differing densities of tau pathology in neuroanatomical regions that correlated with their clinical presentation. In particular, those with corticobasal syndrome had greater tau pathology in the primary motor and somatosensory cortices and putamen, while those with Richardson syndrome had greater tau pathology in limbic and hindbrain structures. Compared with progressive supranuclear palsy, patients with corticobasal degeneration and Richardson syndrome had less neuronal loss in the subthalamic nucleus, but more severe neuronal loss in the medial substantia nigra and greater atrophy of the anterior corpus callosum. Clinically, they had more cognitive impairment and frontal behavioural dysfunction. The results suggest that Richardson syndrome can be a clinicopathological presentation of corticobasal degeneration. Atrophy of anterior corpus callosum may be a potential neuroimaging marker to differentiate corticobasal degeneration from progressive supranuclear palsy in patients with Richardson syndrome.
Figures


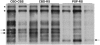
References
-
- Ahmed Z, Josephs KA, Gonzalez J, DelleDonne A, Dickson DW. Clinical and neuropathologic features of progressive supranuclear palsy with severe pallido-nigro-luysial degeneration and axonal dystrophy. Brain. 2008;131:460–72. - PubMed
-
- Arai T, Ikeda K, Akiyama H, Nonaka T, Hasegawa M, Ishiguro K, et al. Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol. 2004;55:72–9. - PubMed
-
- Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–12. - PubMed
-
- Bergeron C, Pollanen MS, Weyer L, Black SE, Lang AE. Unusual clinical presentations of cortical-basal ganglionic degeneration. Ann Neurol. 1996;40:893–900. - PubMed
-
- Boeve BF, Maraganore DM, Parisi JE, Ahlskog JE, Graff-Radford N, Caselli RJ, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology. 1999;53:795–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS057567/NS/NINDS NIH HHS/United States
- RC2-NS070276/NS/NINDS NIH HHS/United States
- R01-AG037491/AG/NIA NIH HHS/United States
- R01 AG024040/AG/NIA NIH HHS/United States
- P50-NS072187/NS/NINDS NIH HHS/United States
- R01-NS057567/NS/NINDS NIH HHS/United States
- P50-AG16574/AG/NIA NIH HHS/United States
- RC2 NS070276/NS/NINDS NIH HHS/United States
- R01 AG037491/AG/NIA NIH HHS/United States
- P01-AG017216/AG/NIA NIH HHS/United States
- P01 AG017216/AG/NIA NIH HHS/United States
- L30 AG051249/AG/NIA NIH HHS/United States
- R01-DC010367/DC/NIDCD NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- R01-AG024040/AG/NIA NIH HHS/United States
- R01 DC010367/DC/NIDCD NIH HHS/United States
- P50-NS72187/NS/NINDS NIH HHS/United States
- P50 NS072187/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

