Two controlled trials to increase participant retention in a randomized controlled trial of mobile phone-based smoking cessation support in the United Kingdom
- PMID: 21933834
- PMCID: PMC3573670
- DOI: 10.1177/1740774511416524
Two controlled trials to increase participant retention in a randomized controlled trial of mobile phone-based smoking cessation support in the United Kingdom
Abstract
Background: Loss to follow-up of trial participants represents a threat to research validity. To date, interventions designed to increase participants' awareness of benefits to society of completing follow-up, and the impact of a telephone call from a senior female clinician and researcher requesting follow-up have not been evaluated robustly.
Purpose: Trial 1 aimed to evaluate the effect on trial follow-up of written information regarding the benefits of participation to society. Trial 2 aimed to evaluate the effect on trial follow-up of a telephone call from a senior female clinician and researcher.
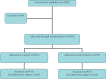
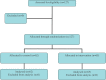
Methods: Two single-blind randomized controlled trials were nested within a larger trial, Txt2stop. In Trial 1, participants were allocated using minimization to receive a refrigerator magnet and a text message emphasizing the benefits to society of completing follow-up, or to a control group receiving a simple reminder regarding follow-up. In Trial 2, participants were randomly allocated to receive a telephone call from a senior female clinician and researcher, or to a control group receiving standard Txt2stop follow-up procedures.
Results: Trial 1: 33.5% (327 of 976) of the intervention group and 33.8% (329 of 974) of the control group returned the questionnaire within 26 weeks of randomization, risk ratio (RR) 0.99; 95% confidence interval (CI) 0.88-1.12. In all, 83.3% (813 of 976) of the intervention group and 82.2% (801 of/974) of the control group sent back the questionnaire within 30 weeks of randomization, RR 1.01; 95% CI 0.97, 1.05. Trial 2: 31% (20 of 65) of the intervention group and 32% (20 of 62) of the control group completed trial follow-up, RR 0.93; 95%CI 0.44, 1.98.
Conclusions: In presence of other methods to increase follow-up neither experimental method (refrigerator magnet and text message emphasizing participation's benefits to society nor a telephone call from study's principal investigator) increased participant follow-up in the Txt2stop trial.
Figures
References
-
- Hunt JR, White E. Retaining and tracking cohort study members. Epidemiol Rev 1998; 20: 57–70 - PubMed
-
- Sprague S, Leece P, Bhandari M, et al. Limiting loss to follow up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials 2003; 24: 719–25 - PubMed
-
- Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002; 359: 781–5 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous