The role of matched sibling donor allogeneic stem cell transplantation in pediatric high-risk acute myeloid leukemia: results from the AML-BFM 98 study
- PMID: 21933851
- PMCID: PMC3248927
- DOI: 10.3324/haematol.2011.051714
The role of matched sibling donor allogeneic stem cell transplantation in pediatric high-risk acute myeloid leukemia: results from the AML-BFM 98 study
Abstract
Background: The role of allogeneic stem cell transplantation in post-remission management of children with high-risk acute myeloid leukemia remains controversial. In the multi-center AML-BFM 98 study we prospectively evaluated the impact of allogeneic stem cell transplantation in children with high-risk acute myeloid leukemia in first complete remission.
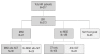
Design and methods: HLA-typed patients with high-risk acute myeloid leukemia, who achieved first complete remission (n = 247), were included in this analysis. All patients received double induction and consolidation. Based on the availability of a matched-sibling donor, patients were allocated by genetic chance to allogeneic stem cell transplantation (n = 61) or chemotherapy-only (i.e. intensification and maintenance therapy; n = 186). The main analysis was done on an intention-to-treat basis according to this allocation.
Results: Intention-to-treat analysis did not show a significantly different 5-year disease-free survival (49 ± 6% versus 45 ± 4%, P(log rank) = 0.44) or overall survival (68 ± 6% versus 57 ± 4%, P(log rank) = 0.17) between the matched-sibling donor and no-matched-sibling donor groups, whereas late adverse effects occurred more frequently after allogeneic stem cell transplantation (72.5% versus 31.8%, P(Fischer)<0.01). These results were confirmed by as-treated analysis corrected for the time until transplantation (5-year overall survival: 72 ± 8% versus 60 ± 4%, P(Mantel-Byar) 0.21). Subgroup analysis demonstrated improved survival rates for patients with 11q23 aberrations allocated to allogeneic stem cell transplantation (5-year overall survival: 94 ± 6% versus 52 ± 7%, P(log-rank) = 0.01; n = 18 versus 49) in contrast to patients without 11q23 aberrations (5-year overall survival: 58 ± 8% versus 55 ± 5%, P(log-rank) = 0.66).
Conclusions: Our analyses defined a genetic subgroup of children with high-risk acute myeloid leukemia who benefited from allogeneic stem cell transplantation in the prospective multi-center AML-BFM 98 study. For the remainder of the pediatric high-risk acute myeloid leukemia patients the prognosis was not improved by allogeneic stem cell transplantation, which was, however, associated with a higher rate of late sequelae.
Figures


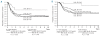
References
-
- Niewerth D, Creutzig U, Bierings MB, Kaspers GJ. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116(13):2205–14. - PubMed
-
- Watson M, Buck G, Wheatley K, Homewood JR, Goldstone AH, Rees JK, et al. Adverse impact of bone marrow transplantation on quality of life in acute myeloid leukaemia patients; analysis of the UK Medical Research Council AML 10 Trial. Eur J Cancer. 2004;40(7):971–8. - PubMed
-
- Leung W, Hudson MM, Strickland DK, Phipps S, Srivastava DK, Ribeiro RC, et al. Late effects of treatment in survivors of childhood acute myeloid leukemia. J Clin Oncol. 2000;18(18):3273–9. - PubMed
-
- Kaspers GJ, Creutzig U. Pediatric acute myeloid leukemia: international progress and future directions. Leukemia. 2005;19(12):2025–9. - PubMed
-
- Michel G, Leverger G, Leblanc T, Nelken B, Baruchel A, Landman-Parker J, et al. Allogeneic bone marrow transplantation vs aggressive post-remission chemotherapy for children with acute myeloid leukemia in first complete remission. A prospective study from the French Society of Pediatric Hematology and Immunology (SHIP) Bone Marrow Transplant. 1996;17(2):191–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

