Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse
- PMID: 21933870
- PMCID: PMC3199004
- DOI: 10.1210/en.2011-1521
Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse
Abstract
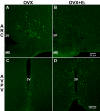
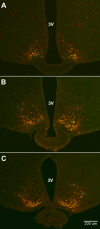
Neurons that produce kisspeptin play a critical role in reproduction. However, understanding the molecular physiology of kisspeptin neurons has been limited by the lack of an in vivo marker for those cells. Here, we report the development of a Kiss1-CreGFP knockin mouse, wherein the endogenous Kiss1 promoter directs the expression of a Cre recombinase-enhanced green fluorescent protein (GFP) fusion protein. The pattern of GFP expression in the brain of the knockin recapitulates what has been described earlier for Kiss1 in the male and female mouse, with prominent expression in the arcuate nucleus (ARC) (in both sexes) and the anteroventral periventricular nucleus (in females). Single-cell RT-PCR showed that the Kiss1 transcript is expressed in 100% of GFP-labeled cells, and the CreGFP transcript was regulated by estradiol in the same manner as the Kiss1 gene (i.e. inhibited in the ARC and induced in the anteroventral periventricular nucleus). We used this mouse to evaluate the biophysical properties of kisspeptin (Kiss1) neurons in the ARC of the female mouse. GFP-expressing Kiss1 neurons were identified in hypothalamic slice preparations of the ARC and patch clamped. Whole-cell (and loose attached) recordings revealed that Kiss1 neurons exhibit spontaneous activity and expressed both h- (pacemaker) and T-type calcium currents, and hyperpolarization-activated cyclic nucleotide-regulated 1-4 and CaV3.1 channel subtypes (measured by single cell RT-PCR), respectively. N-methyl-D-aspartate induced bursting activity, characterized by depolarizing/hyperpolarizing oscillations. Therefore, Kiss1 neurons in the ARC share molecular and electrophysiological properties of other CNS pacemaker neurons.
Figures





References
-
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 - PubMed
-
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 - PubMed
-
- Mikkelsen JD, Simonneaux V. 2009. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides 30:26–33 - PubMed
-
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS43330/NS/NINDS NIH HHS/United States
- R01 HD049651/HD/NICHD NIH HHS/United States
- R01 GM032875/GM/NIGMS NIH HHS/United States
- R01 NS038809/NS/NINDS NIH HHS/United States
- T32 HD007133/HD/NICHD NIH HHS/United States
- T32HD007183/HD/NICHD NIH HHS/United States
- R01 DK068098/DK/NIDDK NIH HHS/United States
- U54 HD012629/HD/NICHD NIH HHS/United States
- T32 HD007183/HD/NICHD NIH HHS/United States
- U54 HD12 629/HD/NICHD NIH HHS/United States
- R01 NS38809/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- MH086386/MH/NIMH NIH HHS/United States
- R01 NS043330/NS/NINDS NIH HHS/United States
- R01 MH086386/MH/NIMH NIH HHS/United States
- R01 DK68098/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

