Hepatocyte entry leads to degradation of autoreactive CD8 T cells
- PMID: 21933957
- PMCID: PMC3189041
- DOI: 10.1073/pnas.1112251108
Hepatocyte entry leads to degradation of autoreactive CD8 T cells
Abstract
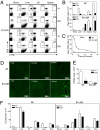
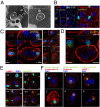
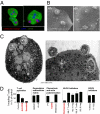
Although most self-reactive T cells are eliminated in the thymus, mechanisms to inactivate or control T cells specific for extrathymic antigens are required and exist in the periphery. By investigating the site in which autoreactive T cells are tolerized, we identify a unique mechanism of peripheral deletion in which naïve autoreactive CD8 T cells are rapidly eliminated in the liver after intrahepatic activation. T cells actively invade hepatocytes, enter endosomal/lysosomal compartments, and are degraded. Blockade of this process leads to accumulation of autoreactive CD8 T cells in the liver and breach of tolerance, with the development of autoimmune hepatitis. Cell into cell invasion, or emperipolesis, is a long-observed phenomenon for which a physiological role has not been previously demonstrated. We propose that this "suicidal emperipolesis" is a unique mechanism of autoreactive T-cell deletion, a process critical for the maintenance of tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Immune tolerance: Suicide is painless.Nat Rev Immunol. 2011 Oct 14;11(11):719. doi: 10.1038/nri3092. Nat Rev Immunol. 2011. PMID: 21997791 No abstract available.
References
-
- Sprent J, Kishimoto H. The thymus and negative selection. Immunol Rev. 2002;185:126–135. - PubMed
-
- Srinivasan M, Frauwirth KA. Peripheral tolerance in CD8+ T cells. Cytokine. 2009;46:147–159. - PubMed
-
- Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. - PubMed
-
- Feuerer M, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–1157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

