Contraceptive choices, pregnancy rates, and outcomes in a microbicide trial
- PMID: 21934454
- PMCID: PMC3178043
- DOI: 10.1097/AOG.0b013e31822be512
Contraceptive choices, pregnancy rates, and outcomes in a microbicide trial
Abstract
Objective: Women who become pregnant during the conduct of biomedical human immunodeficiency virus prevention trials are taken off the study product for safety reasons. High pregnancy rates can compromise statistical integrity in these trials. The comprehensive contraceptive curriculum developed for the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 004 trial was evaluated for its ability to enhance contraceptive uptake, reduce pregnancy rates, and preserve statistical integrity.
Methods: Contraceptive- and pregnancy-related eligibility criteria were specified in the protocol. We enrolled women who opted for a nonbarrier method of contraceptive and provided hormonal contraceptives onsite at no cost. At each monthly study visit, we provided pregnancy prevention counseling and performed pregnancy testing. Study product was withheld on pregnancy diagnosis, but women continued with monthly follow-up.
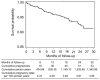
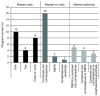
Results: Contraceptive use was high throughout the study with 100% uptake at baseline and 94.71% use after a mean of 18 months follow-up at exit. Injectable progestins, particularly medroxyprogesterone acetate, remained the preferred choice of contraceptive. After 30 months of follow-up, 54 pregnancies were reported out of 889 participants, giving a pregnancy incidence rate of 3.95 per 100 woman-years (95% confidence interval 2.96-5.17). Of all pregnancies, two thirds (64.81%) resulted in a full-term live birth, whereas 18.52% and 11.11% pregnancies culminated as miscarriage and terminated pregnancies, respectively. There were no congenital anomalies in the early neonatal period. Pregnancies resulted in 1.56% of woman-years of study follow-up lost as a result of temporary product withdrawal.
Conclusion: The CAPRISA 004 contraceptive curriculum was an effective strategy for maintaining low pregnancy rates, thereby minimizing product withdrawal and loss of follow-up time.
Level of evidence: III.
Figures

References
-
- UNAIDS . UNAIDS Report on the global AIDS Epidemic 2010. Joint United Nations Programme on HIV/AIDS; Geneva: [Accessed 11 February 2011]. 2010. Available from: http://www.unaids.org/globalreport/
-
- UNAIDS . Report on the global AIDS Epidemic Update. Joint United Nations Programme on HIV/AIDS; Geneva: [Accessed 31 October 2008]. 2008. http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_.... 2008.
-
- AVAC [Accessed February 2011]];Ongoing and planned PreExposure Prophylaxis (PrEP) trials (Feb 2011) 2011 Available from: http://www.avac.org/ht/a/GetDocumentAction/i/3113.
-
- Raymond EG, Taylor D, Cates W, Jr., Tolley EE, Borasky D, Cancel A, et al. Pregnancy in effectiveness trials of HIV prevention agents. Sexually transmitted diseases. 2007 Dec;34(12):1035–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

