The phenotypic radiation resistance of CD44+/CD24(-or low) breast cancer cells is mediated through the enhanced activation of ATM signaling
- PMID: 21935375
- PMCID: PMC3174160
- DOI: 10.1371/journal.pone.0024080
The phenotypic radiation resistance of CD44+/CD24(-or low) breast cancer cells is mediated through the enhanced activation of ATM signaling
Abstract
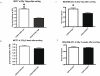
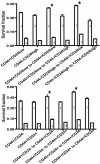
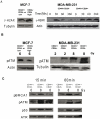
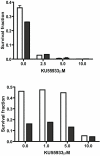
Cancer initiating cells (CIC) are stem-like cells. CIC may contribute not only to the initiation of cancer but also to cancer recurrence because of the resistance of CIC both to chemotherapy and radiation therapy. From the MCF-7 and MDA-MB231 breast cancer cell lines and primary culture of patient breast cancer cells, we isolated by flow cytometry a CIC subset of cells with the CD44(+)/CD24(-or low) phenotype. The CD44(+)/CD24(-or low) subset showed increased sphere formation and resistance to radiation compared to the non- CD44(+)/CD24(-or low) subset. The increased radiation resistance was not dependent on the result of altered non-homologous end joining (NHEJ) DNA repair activity as both NHEJ activity and expression of the various proteins involved in NHEJ were not significantly different between the CD44(+)/CD24(-or low) and non- CD44(+)/CD24(-or low) subsets. However, activation of ATM signaling was significantly increased in CD44(+)/CD24(-or low) cells compared to non- CD44(+)/CD24(-or low) cells in both from breast cancer cell lines and primary human breast cancer cells. Application of an ATM inhibitor effectively decreased the radiation resistance of CD44(+)/CD24(-or low) subset, suggesting that targeting ATM signaling may provide a new tool to eradicate stem-like CIC and abolish the radiation resistance of breast cancer.
Conflict of interest statement
Figures



References
-
- Ponti D, Zaffaroni N, Capelli C, Daidone MG. Breast cancer stem cells: an overview. Eur J Cancer. 2006;42:1219–1224. - PubMed
-
- Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. J Cell Sci. 2005;118:3585–3594. - PubMed
-
- Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. - PubMed
-
- Giatromanolaki A, Sivridis E, Fiska A, Koukourakis MI. The CD44+/CD24− phenotype relates to ‘triple-negative’ state and unfavorable prognosis in breast cancer patients. Med Oncol. 2011;28:745–752. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

