SPL7013 Gel (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans
- PMID: 21935377
- PMCID: PMC3174146
- DOI: 10.1371/journal.pone.0024095
SPL7013 Gel (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans
Abstract
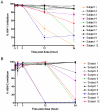
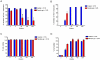
SPL7013 Gel (VivaGel(®)) is a microbicide in development for prevention of HIV and HSV. This clinical study assessed retention and duration of antiviral activity following vaginal administration of 3% SPL7013 Gel in healthy women. Participants received 5 single doses of product with ≥5 days between doses. A cervicovaginal fluid (CVF) sample was collected using a SoftCup™ pre-dose, and immediately, or 1, 3, 12 or 24 h post-dose. HIV-1 and HSV-2 antiviral activities of CVF samples were determined in cell culture assays. Antiviral activity in the presence of seminal plasma was also tested. Mass and concentration of SPL7013 in CVF samples was determined. Safety was assessed by reporting of adverse events. Statistical analysis was performed using the Wilcoxon signed-rank test with Bonferroni adjustment; p≤0.003 was significant. Eleven participants completed the study. Inhibition of HIV-1 and HSV-2 by pre-dose CVF samples was negligible. CVF samples obtained immediately after dosing almost completely inhibited (median, interquartile range) HIV-1 [96% (95,97)] and HSV-2 [86% (85,94)], and activity was maintained in all women at 3 h (HIV-1 [96% (95,98), p = 0.9]; HSV-2 [94% (91,97), p = 0.005]). At 24 h, >90% of initial HIV-1 and HSV-2 inhibition was maintained in 6/11 women. SPL7013 was recovered in CVF samples obtained at baseline (46% of 105 mg dose). At 3 and 24 h, 22 mg and 4 mg SPL7013, respectively, were recovered. More than 70% inhibition of HIV-1 and HSV-2 was observed if there was >0.5 mg SPL7013 in CVF samples. High levels of antiviral activity were retained in the presence of seminal plasma. VivaGel was well tolerated with no signs or symptoms of vaginal, vulvar or cervical irritation reported. Potent antiviral activity was observed against HIV-1 and HSV-2 immediately following vaginal administration of VivaGel, with activity maintained for at least 3 h post-dose. The data provide evidence of antiviral activity in a clinical setting, and suggest VivaGel could be administered up to 3 h before coitus.
Trial registration: The study is registered at ClinicalTrials.gov under identifier: NCT00740584.
Conflict of interest statement
Figures




Similar articles
-
VivaGel (SPL7013 Gel): a candidate dendrimer--microbicide for the prevention of HIV and HSV infection.Int J Nanomedicine. 2007;2(4):561-6. Int J Nanomedicine. 2007. PMID: 18203424 Free PMC article. Review.
-
Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004).AIDS. 2011 May 15;25(8):1057-64. doi: 10.1097/QAD.0b013e328346bd3e. AIDS. 2011. PMID: 21505316 Free PMC article. Clinical Trial.
-
Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses.Antiviral Res. 2005 Dec;68(3):139-46. doi: 10.1016/j.antiviral.2005.08.004. Epub 2005 Sep 27. Antiviral Res. 2005. PMID: 16219368
-
Structure activity relationship of dendrimer microbicides with dual action antiviral activity.PLoS One. 2010 Aug 23;5(8):e12309. doi: 10.1371/journal.pone.0012309. PLoS One. 2010. PMID: 20808791 Free PMC article.
-
Mechanistic Studies of Viral Entry: An Overview of Dendrimer-Based Microbicides As Entry Inhibitors Against Both HIV and HSV-2 Overlapped Infections.Med Res Rev. 2017 Jan;37(1):149-179. doi: 10.1002/med.21405. Epub 2016 Aug 12. Med Res Rev. 2017. PMID: 27518199 Review.
Cited by
-
Controlling the HIV/AIDS epidemic: current status and global challenges.Front Immunol. 2012 Aug 14;3:250. doi: 10.3389/fimmu.2012.00250. eCollection 2012. Front Immunol. 2012. PMID: 22912636 Free PMC article.
-
Optimal management of genital herpes: current perspectives.Infect Drug Resist. 2016 Jun 13;9:129-41. doi: 10.2147/IDR.S96164. eCollection 2016. Infect Drug Resist. 2016. PMID: 27358569 Free PMC article. Review.
-
Nanoparticles as potential new generation broad spectrum antimicrobial agents.Daru. 2015 Sep 2;23:43. doi: 10.1186/s40199-015-0125-6. Daru. 2015. PMID: 26329777 Free PMC article. Review.
-
Approved Nanomedicine against Diseases.Pharmaceutics. 2023 Feb 26;15(3):774. doi: 10.3390/pharmaceutics15030774. Pharmaceutics. 2023. PMID: 36986635 Free PMC article. Review.
-
Dendrimers as potential therapeutic tools in HIV inhibition.Molecules. 2013 Jul 5;18(7):7912-29. doi: 10.3390/molecules18077912. Molecules. 2013. PMID: 23884127 Free PMC article. Review.
References
-
- UNAIDS . Report on the global AIDS epidemic; 2008. Joint United Nations Programme on HIV/AIDS (UNAIDS).
-
- Balzarini J, Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007;369:787–797. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

