Active zone density is conserved during synaptic growth but impaired in aged mice
- PMID: 21935939
- PMCID: PMC3731066
- DOI: 10.1002/cne.22764
Active zone density is conserved during synaptic growth but impaired in aged mice
Abstract
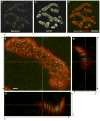
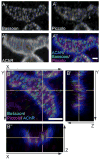
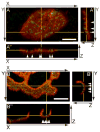
Presynaptic active zones are essential structures for synaptic vesicle release, but the developmental regulation of their number and maintenance during aging at mammalian neuromuscular junctions (NMJs) remains unknown. Here, we analyzed the distribution of active zones in developing, mature, and aged mouse NMJs by immunohistochemical detection of the active zone-specific protein Bassoon. Bassoon is a cytosolic scaffolding protein essential for the active zone assembly in ribbon synapses and some brain synapses. Bassoon staining showed a punctate pattern in nerve terminals and axons at the nascent NMJ on embryonic days 16.5-18.5. Three-dimensional reconstruction of NMJs revealed that the majority of Bassoon puncta within an NMJ were attached to the presynaptic membrane from postnatal day 0 to adulthood, and colocalized with another active zone protein, Piccolo. During postnatal development, the number of Bassoon puncta increased as the size of the synapses increased. Importantly, the density of Bassoon puncta remained relatively constant from postnatal day 0 to 54 at 2.3 puncta/μm(2) , while the synapse size increased 3.3-fold. However, Bassoon puncta density and signal intensity were significantly attenuated at the NMJs of 27-month-old aged mice. These results suggest that synapses maintain the density of synaptic vesicle release sites while the synapse size changes, but this density becomes impaired during aging.
Copyright © 2011 Wiley-Liss, Inc.
Conflict of interest statement
The authors have no competing interests to declare.
Figures





References
-
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3(5):445–451. - PubMed
-
- Alshuaib WB, Fahim MA. Depolarization reverses age-related decrease of spontaneous transmitter release. J Appl Physiol. 1991;70(5):2066–2071. - PubMed
-
- Balice-Gordon RJ. Age-related changes in neuromuscular innervation. Muscle Nerve Suppl. 1997;5:S83–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

