Pre-Bötzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons
- PMID: 21935944
- PMCID: PMC3925347
- DOI: 10.1002/cne.22769
Pre-Bötzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons
Abstract
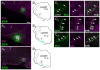
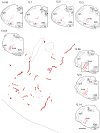
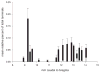
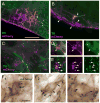
The retrotrapezoid nucleus (RTN) contains CO(2) -responsive neurons that regulate breathing frequency and amplitude. These neurons (RTN-Phox2b neurons) contain the transcription factor Phox2b, vesicular glutamate transporter 2 (VGLUT2) mRNA, and a subset contains preprogalanin mRNA. We wished to determine whether the terminals of RTN-Phox2b neurons contain galanin and VGLUT2 proteins, to identify the specific projections of the galaninergic subset, to test whether RTN-Phox2b neurons contact neurons in the pre-Bötzinger complex, and to identify the ultrastructure of these synapses. The axonal projections of RTN-Phox2b neurons were traced by using biotinylated dextran amine (BDA), and many BDA-ir boutons were found to contain galanin immunoreactivity. RTN galaninergic neurons had ipsilateral projections that were identical with those of this nucleus at large: the ventral respiratory column, the caudolateral nucleus of the solitary tract, and the pontine Kölliker-Fuse, intertrigeminal region, and lateral parabrachial nucleus. For ultrastructural studies, RTN-Phox2b neurons (galaninergic and others) were transfected with a lentiviral vector that expresses mCherry almost exclusively in Phox2b-ir neurons. After spinal cord injections of a catecholamine neuron-selective toxin, there was a depletion of C1 neurons in the RTN area; thus it was determined that the mCherry-positive terminals located in the pre-Bötzinger complex originated almost exclusively from the RTN-Phox2b (non-C1) neurons. These terminals were generally VGLUT2-immunoreactive and formed numerous close appositions with neurokinin-1 receptor-ir pre-Bötzinger complex neurons. Their boutons (n = 48) formed asymmetric synapses filled with small clear vesicles. In summary, RTN-Phox2b neurons, including the galaninergic subset, selectively innervate the respiratory pattern generator plus a portion of the dorsolateral pons. RTN-Phox2b neurons establish classic excitatory glutamatergic synapses with pre-Bötzinger complex neurons presumed to generate the respiratory rhythm.
Copyright © 2011 Wiley-Liss, Inc.
Figures



References
-
- Abbott SB, Burke PG, Pilowsky PM. Galanin microinjection into the PreBötzinger or the Bötzinger complex terminates central inspiratory activity and reduces responses to hypoxia and hypercapnia in rat. Respir Physiol Neurobiol. 2009a;167:299–306. - PubMed
-
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol. 2002;31:693–717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

